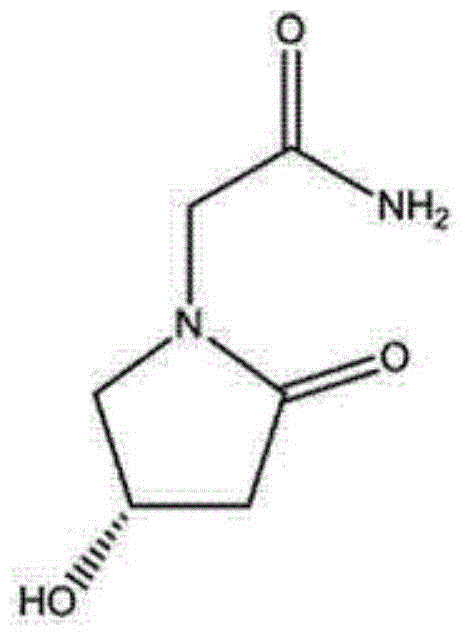
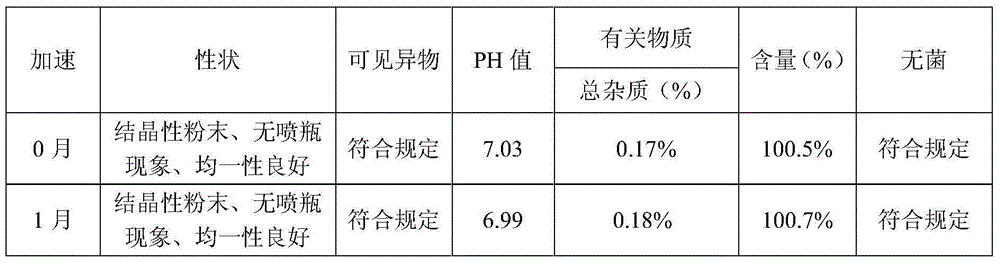
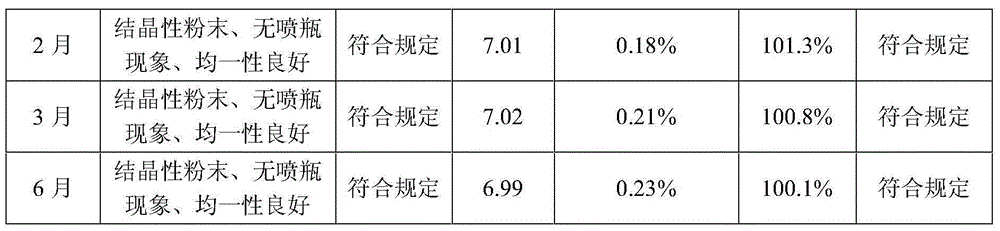
Levo oxiracetam lyophilized powder for injection and preparation method thereof
A technology of freeze-dried powder injection and freeze-drying, which is applied in the direction of freeze-drying transportation, powder transportation, and pharmaceutical formulations. It can solve the problems of inconsistent properties of the upper and lower layers, poor product uniformity, and difficulty in forming a skeleton, and achieve consistent properties of the upper and lower layers. , Reduce adverse drug reactions, good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0023] Element Dosage Levoxiracetam 100g L-serine 70g Mannitol 85g sodium glutamate 15g
[0024] Makes 1000 bottles
[0025] Preparation process:
[0026] 1. Concentrated preparation: put the prescribed amount of levoxiracetam and excipients in a container, add 5 times the weight of levoxiracetam and sterilized water for injection to stir, after dissolving, add 0.5% by mass fraction of the needle Stir with activated carbon for 30 minutes, then filter with a 0.45 micron microporous membrane, collect the filtrate, and set aside;
[0027] 2. Dilute preparation: add sterile water for injection to the filtrate to the prescribed amount, adjust the pH to 7.0 with hydrochloric acid or sodium hydroxide, then sterilize and filter with a 0.22-micron microporous membrane, take the filtrate and fill it in In a sterile glass bottle, spare;
[0028] 3....
Embodiment 2
[0031] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0032] Element Dosage Levoxiracetam 100g L-serine 55g Mannitol 72g sodium glutamate 12g
[0033] Makes 1000 bottles
[0034] Preparation process: prepared according to the preparation process of Example 1.
Embodiment 3
[0036] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0037] Element Dosage Levoxiracetam 100g L-serine 60g
[0038] Mannitol 75g sodium glutamate 15g
[0039] Makes 1000 bottles
[0040] Preparation process: prepared according to the preparation process of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com