(S)-4-hydroxyl-2 oxo-1-pyrrolidine acetamide freeze-drying powder for injection and preparation method of freeze-drying powder
A technology for pyrrolidine acetamide and injection, which is applied in freeze-dried delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as poor stability, short shelf life, and no fixed shape. , to achieve the effect of good clarity, good stability and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder for injection, prepared according to the following steps:
[0021] Element
[0022] Makes 1000 bottles
[0023] Preparation process:
[0024] 1. Concentrated preparation: put the raw and auxiliary materials of the prescribed amount in a container, add (S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide 10 times the weight of sterile water for injection and stir, after dissolving, add Activated carbon for needles with a mass fraction of 0.5% was stirred for 30 minutes, then filtered through a 0.45 micron microporous membrane, and the filtrate was collected for subsequent use;
[0025] 2. Dilute preparation: add sterilized water for injection to the filtrate to 1000 times the volume of the filtrate, adjust the pH to 5.5 with hydrochloric acid or sodium hydroxide, then sterilize and filter with a 0.22 micron microporous membrane, take the filtrate and fill it Divide into sterile glass bottles and set asid...
Embodiment 2
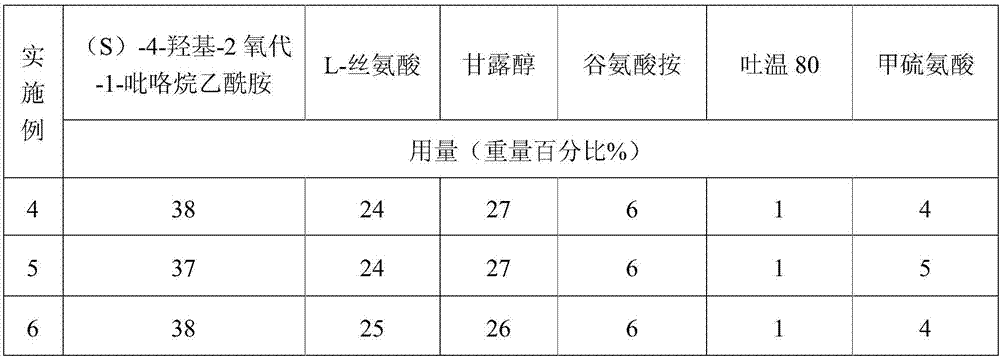
[0065] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder for injection, prepared according to the following steps:
[0066] Element
Dosage (% by weight)
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
39%
L-serine
24%
26%
sodium glutamate
6%
Tween 80
1%
4%
[0067] Makes 1000 bottles
[0068] Preparation process: prepared according to the preparation process of Example 1.
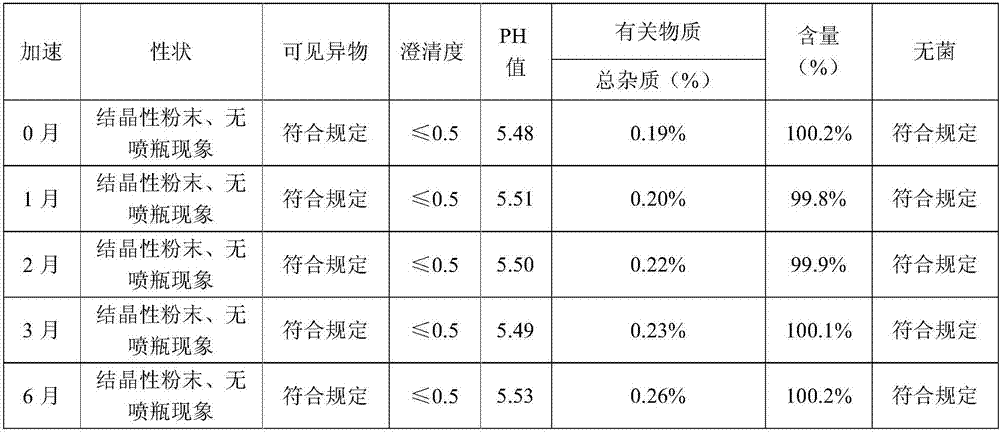
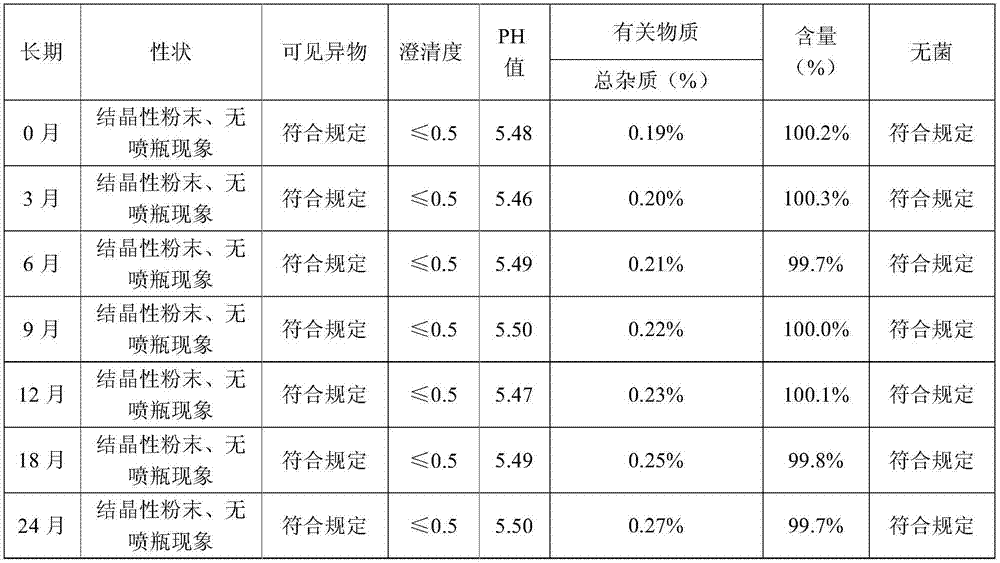
[0069] Press the test method of embodiment 1, respectively carry out stability test, spray bottle phenomenon statistical experiment and preparation process to the impact experiment of impurity increase in the freeze-drying process, the sample stability test result of embodiment 2 shows that accelerated June sample quality is stable, long-term The quality is stable for 24 months, so the validity period of this product is at least 24 months; the sample of Example 2 did not spray the bottle during th...
Embodiment 3
[0071] A (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide freeze-dried powder for injection, prepared according to the following steps:
[0072] Element
Dosage (% by weight)
(S)-4-Hydroxy-2-oxo-1-pyrrolidineacetamide
37%
L-serine
25%
26%
6%
Tween 80
2%
4%
[0073] Makes 1000 bottles
[0074] Preparation process: prepared according to the preparation process of Example 1.
[0075] By the test method of embodiment 1, respectively carry out stability test, spray bottle phenomenon statistical experiment and preparation process to the impact experiment of impurity increase in the freeze-drying process, embodiment 3 sample stability test results show that accelerated June sample quality is stable, long-term The quality is stable for 24 months, so the valid period of this product is at least 24 months; the sample of Example 3 has no bottle spray phenomenon during the freez...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com