Preparation method of L-oxiracetam freeze-dried powder
A freeze-dried powder and drying technology, which is applied in the direction of freeze-dried transportation, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of poor product uniformity, inconsistent properties of the upper and lower layers, and poor product stability. To achieve the effect of reducing adverse drug reactions, consistent properties of the upper and lower layers, and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
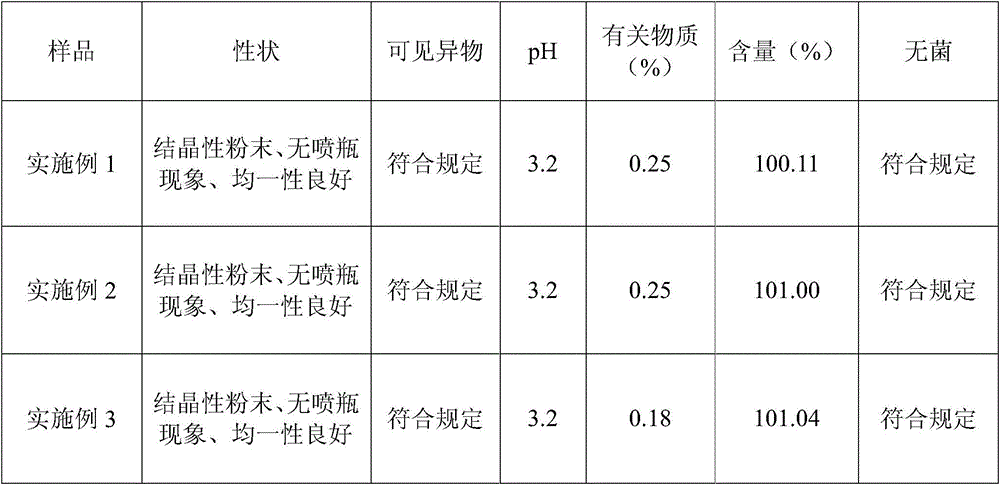
Embodiment 1
[0015] The prescription of the levoxiracetam lyophilized powder of embodiment 1 is shown in the table below:
[0016] prescription
weight percentage
Levoxiracetam
50%
L-serine
25%
14.9%
sodium glutamate
5%
5%
0.1%
[0017] The preparation method of the levoxiracetam freeze-dried powder of embodiment 1 may further comprise the steps:
[0018] (1) Concentrated preparation: put the raw and auxiliary materials of the prescribed amount in a container, add sterilized water for injection with 10 times the weight of levoxiracetam and stir, after dissolving, add activated carbon for needles with a mass fraction of 0.1%, and stir for 30 minutes. Then filter with a 0.45 micron microporous membrane, collect the filtrate, and set aside;
[0019] (2) Dilute preparation: add sterile water for injection to the filtrate to 1000 times the volume of the filtrate, adjust the pH value ...
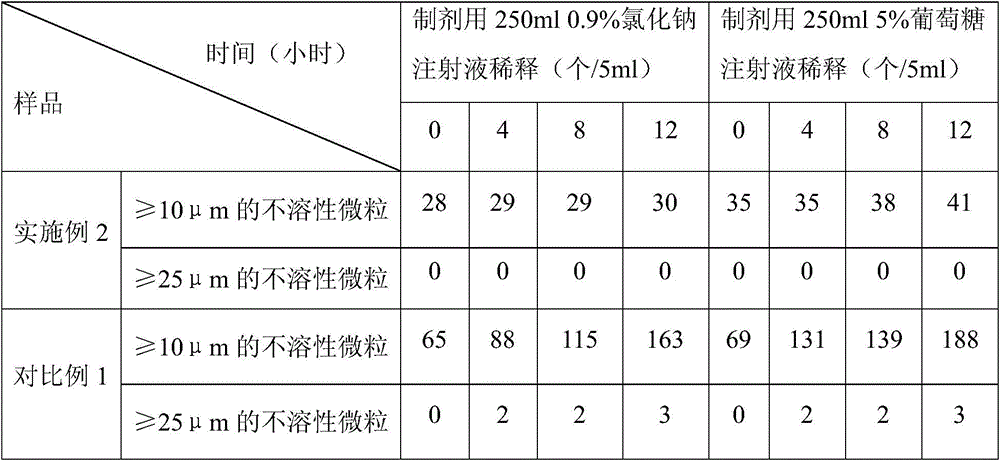
Embodiment 2
[0023] The prescription of the levoxiracetam lyophilized powder of embodiment 2 is shown in the table below:
[0024] prescription
[0025] The preparation method of the levoxiracetam freeze-dried powder of embodiment 2 is the same as that of embodiment 1.
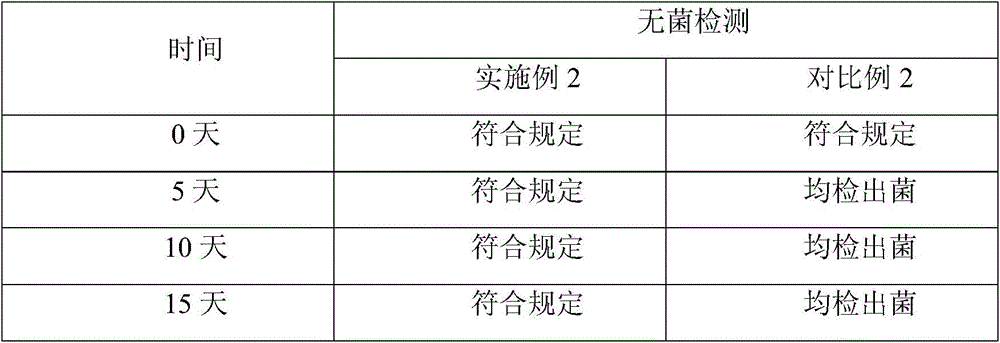
Embodiment 3
[0027] The prescription of the levoxiracetam freeze-dried powder of embodiment 3 is shown in the table below:
[0028] prescription
[0029] The preparation method of the levoxiracetam freeze-dried powder of embodiment 3 is the same as that of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com