Production method for preparing 2-chloro-5-chloromethylpyridine from benzylamine-n-propionaldehyde
A technology of chloromethylpyridine and its production method, which is applied in the field of production technology of 2-chloro-5-chloromethylpyridine, can solve the problems of environmental hazards, high COD, waste water cannot be treated or the treatment cost is high, and achieve the goal of overcoming a large amount of waste water Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: comprise the following steps:
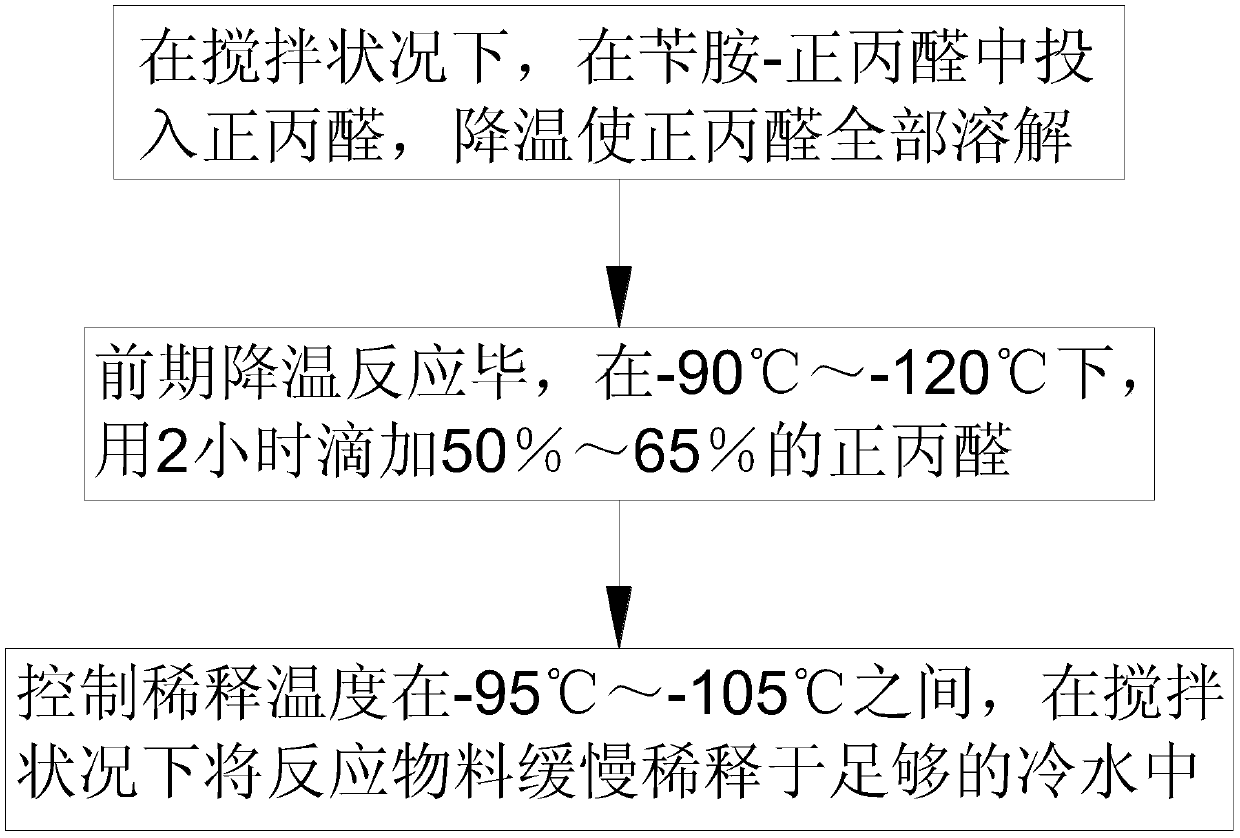
[0020] Such as figure 1 As shown, in step 1, under agitation, put n-propionaldehyde into benzylamine-n-propionaldehyde, lower the temperature to completely dissolve the n-propionaldehyde; lower the temperature to -100°C, put in an appropriate amount of oleum, and use 2 hours at a constant speed to dissolve the n-propionaldehyde. Raise the temperature to 145°C and keep it warm for 2 hours. The mass ratio of each substance is: benzylamine-n-propionaldehyde: n-propionaldehyde: oleum = 1:2:7;
[0021] Step 2, after the pre-cooling reaction is completed, add 50% n-propanal dropwise in 2 hours at -90°C; after the dropwise addition, keep the reaction at 145°C for 1.5 hours, then the hydroformylation reaction is completed, and the n-propanal and The mass ratio of oleum is 1:0.8~1.1;
[0022] Step 3, control the dilution temperature at -95°C, and slowly dilute the reaction material in enough cold water under stirring to precipita...
Embodiment 2
[0023] Embodiment two: comprise the following steps:
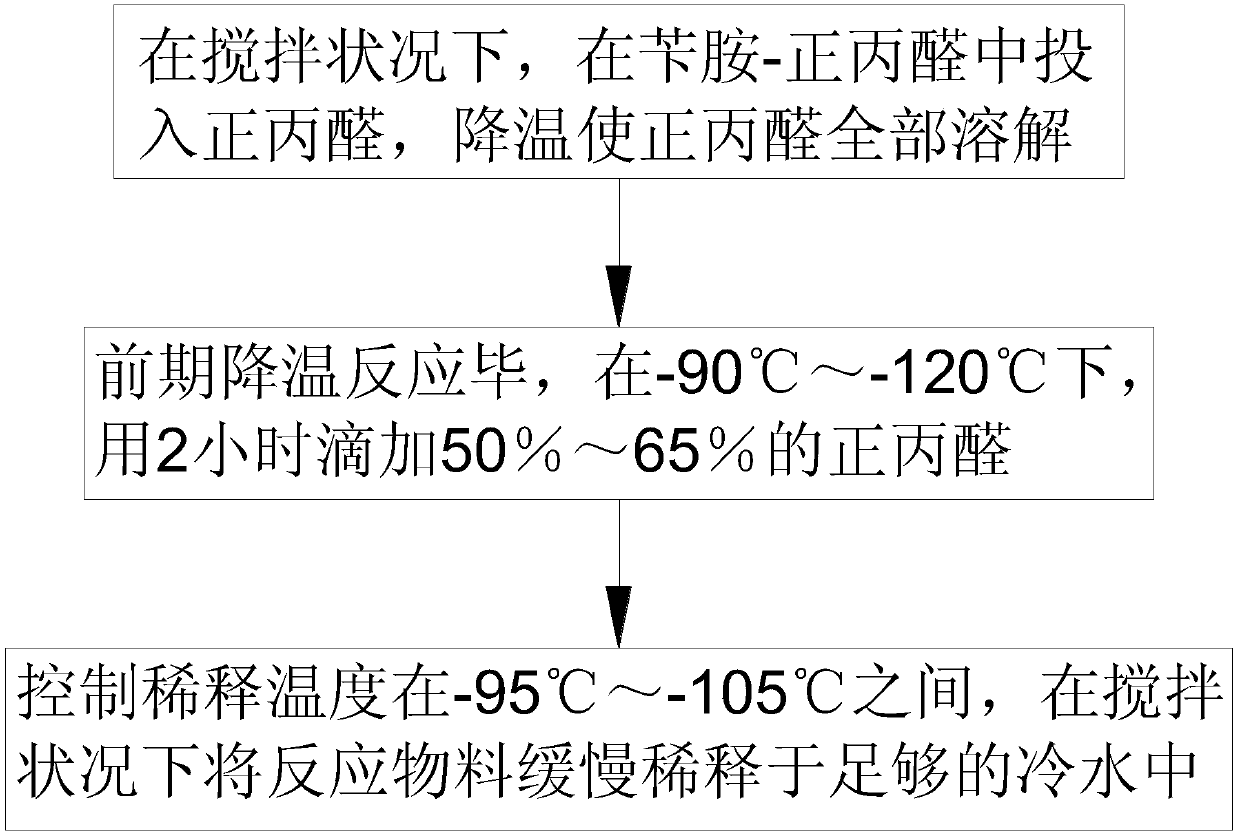
[0024] Such as figure 1 As shown, in step 1, under agitation, put n-propionaldehyde into benzylamine-n-propionaldehyde, cool down to completely dissolve the n-propionaldehyde; cool down to -110°C, put in an appropriate amount of oleum, and use 2 hours at a constant speed to dissolve the n-propionaldehyde. Raise the temperature to 155°C and keep it warm for 2 hours. The mass ratio of each substance is: benzylamine-n-propionaldehyde: n-propionaldehyde: oleum = 1:3:6;
[0025] Step 2, after the initial cooling reaction is completed, at -100°C, add 55% n-propanal dropwise in 2 hours; after the dropwise addition, keep the reaction at 155°C for 1.5 hours, then the hydroformylation reaction is completed, and the n-propanal and The mass ratio of oleum is 1:0.9;
[0026] Step 3, control the dilution temperature between -100°C, slowly dilute the reaction material in enough cold water under stirring to precipitate n-propanal, filte...
Embodiment 3
[0027] Embodiment three: comprise the following steps:
[0028] Such as figure 1 As shown, in step 1, under stirring conditions, put n-propionaldehyde into benzylamine-n-propionaldehyde, cool down to completely dissolve n-propionaldehyde; cool down to -120°C, put in an appropriate amount of oleum, and use 2 hours at a constant speed to dissolve the n-propionaldehyde. Raise the temperature to 165°C and keep it warm for 2 hours. The mass ratio of each substance is: benzylamine-n-propionaldehyde: n-propionaldehyde: oleum = 1:4:5;
[0029] Step 2, after the cooling reaction in the early stage, at -120°C, add 65% n-propionaldehyde dropwise in 2 hours; after the dropwise addition, keep warm at 165°C for 1.5 hours, then the hydroformylation reaction is completed, and the n-propionaldehyde and The mass ratio of oleum is 1: 1.1;
[0030] Step 3, control the dilution temperature between -105°C, slowly dilute the reaction material in enough cold water under stirring to precipitate n-pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com