Serotonin 5-HI1A and dopamin D2 receptor ligands
A hydroxyl, phenylpiperazine technology, applied in the field of serotonin 5-HT1A and dopamine D2 receptor ligands, can solve the problem of not providing test data and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
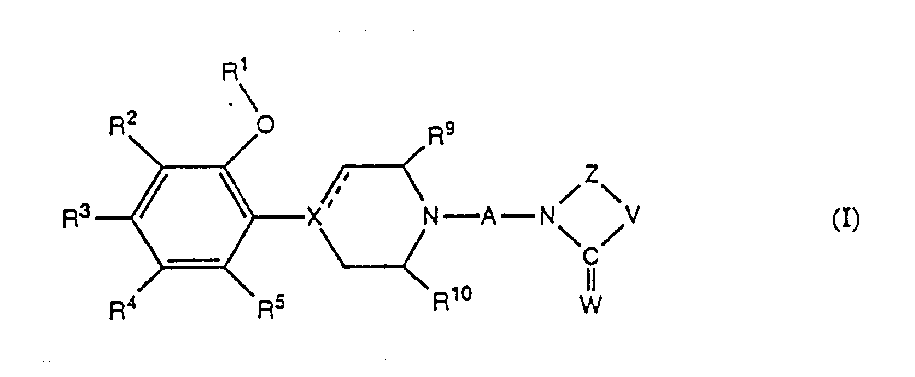
[0039] The present invention further relates to the preparation method of the new 4-phenylpiperazine compound of formula I, 4-phenylpiperidine compound and 4-phenyl-1,2,3,6-tetrahydropyridine compound, The method comprises: a) reacting a compound of formula II with a compound of formula III: where R 1 -R 5 , R 9 , R 10 , X, V, W, Z, n and dashed lines are as defined above, Y represents a suitable leaving group such as halogen, mesylate or tosylate; or b) reducing the amide carbonyl of formula IV Compound: where R 1 -R 5 , R 9 , R 10 , X, V, W, Z, n and dashed lines are as defined above; or c) make the compound of formula V where R 2 -R 5 , R 9 , R10 , X, V, W, Z, A and dashed lines are defined as above, with compound R 1 Y response, where R 1 is defined above and Y represents a suitable leaving group such as a halogen, mesylate or tosylate; or d) with an aldehyde R'CHO, a ketone R"R_CO or a carboxylic acid R'COOH (where R', R" and R_ are together with the nit...
Embodiment 1
[0052] Example 1 (similar method d) 1-(4-chloro-1-butyl)-3-cyclohexyl-2-imidazolidinone 1a
[0053] A mixture of 1-(4-chloro-1-butyl)-2-imidazolidinone (50 g) and cyclohexanone (83.3 g) in glacial acetic acid (1000 ml) was stirred at room temperature for 1 hour and the resulting mixture was cooled to 10-15°C and add NaBH in small batches within 5 hours 4 (42.4g). After stirring at room temperature overnight, the acetic acid was evaporated in vacuo. Add water (500ml) and dichloromethane (300ml) and by adding dilute NH 4 Aqueous OH solution adjusted the pH to greater than 9. The organic phase was separated, dried over anhydrous magnesium sulfate, filtered and the solvent was evaporated in vacuo. The residual crude product was purified by silica gel column chromatography (eluting with ethyl acetate). Pure title compound 1a crystallized on standing. Yield: 33 g, mp: 30-35°C.
[0054] The following imidazolidinones were prepared according to the corresponding method: 1-(3-Ch...
Embodiment 2
[0055] Example 21-(4-chloro-1-butyl)-3-(4-fluorophenyl)-2-imidazolidinone 2a
[0056] To a solution of 2-aminoethanol (1100 g) in ethanol (1000 ml) was added 4-chlorobutanol (220 g), and the resulting mixture was refluxed for 4 hours. After cooling to 10°C, a solution of sodium methoxide in methanol (380ml) was added. The precipitate was filtered off and the volatiles were evaporated in vacuo. The remaining oil was distilled under reduced pressure. 4-[N-(2-Hydroxyethyl)amino]butanol was collected at 135-140° C. at 0.2-0.4 mmHg, yield: 106 g. Amino alcohol (25g) was dissolved in dichloromethane (150ml) and a solution of 4-fluorophenylisocyanate (26g) in dichloromethane (25ml) was added dropwise at 0-8°C. After reflux for 2 hours, the solvent was removed in vacuo to give crude 1-(2-hydroxyethyl)-1-(4-hydroxybutyl)-3-(4-fluorophenyl)urea as an oil, yield: 56 g . To a solution of all the crude urea derivatives and N,N-dimethylformamide (DMF, 1 ml) in dichloromethane (250 ml) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com