Triazolopyrazinones as PDE1 inhibitors
A technology of triazolo and pyrazine, which is applied in the field of triazolopyrazinone as a PDE1 inhibitor, and can solve problems such as not disclosing it
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
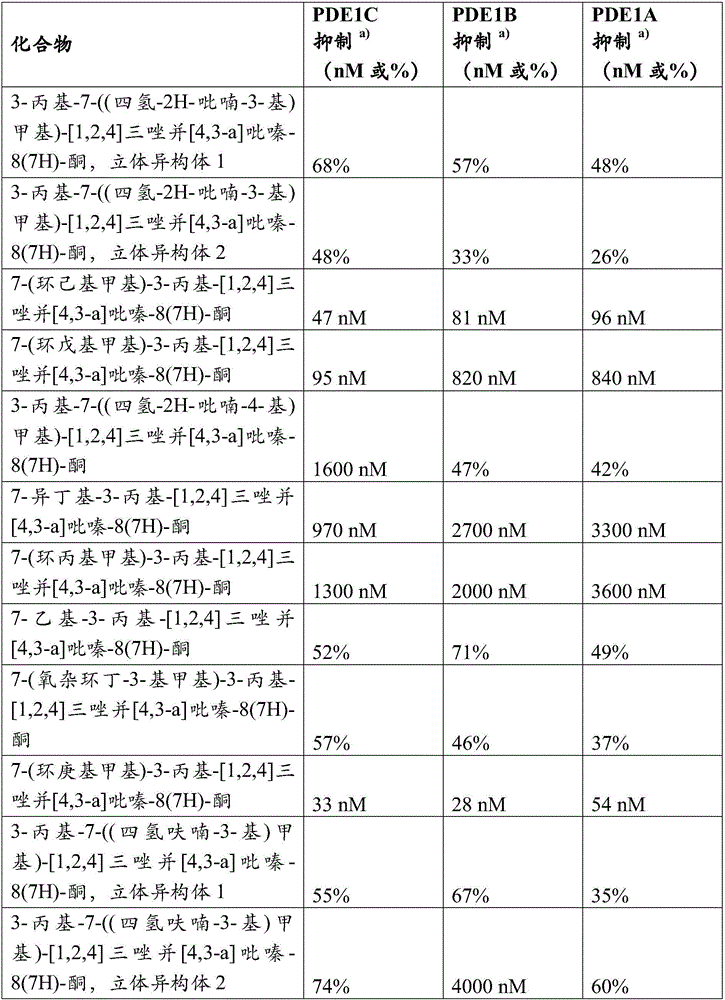
Examples
no. 1 example E1
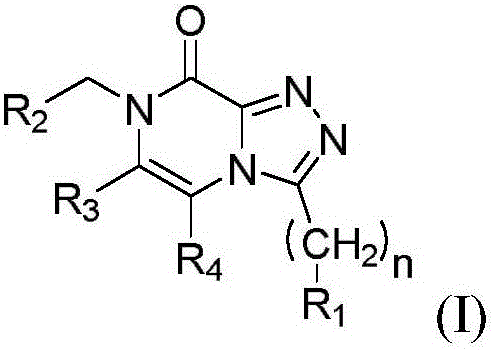
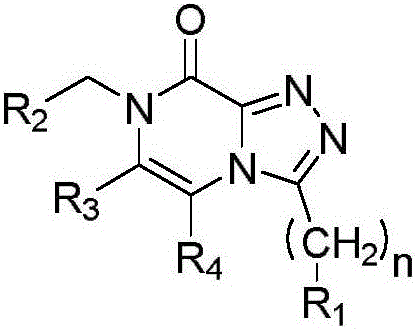
[0031] In a first embodiment E1, the invention relates to a compound (compound I) of formula (I):
[0032]
[0033] Compound (I)
[0034] in
[0035] n is 0 or 1;
[0036] R 1 selected from the group consisting of: linear or branched C 1 -C 8 Alkyl, such as C 1 to C 3 Alkyl, oxetanyl, tetrahydrofuranyl, tetrahydropyranyl and saturated monocyclic C 3 -C 8 Cycloalkyl; or
[0037] R 1 selected from the group consisting of oxetanyl, tetrahydrofuranyl, tetrahydropyranyl, saturated monocyclic C 3 -C 8 Cycloalkyl, and substituted by fluorine one or more times, preferably once, twice or three times, straight or branched chain C 1 -C 8 alkyl; and
[0038] R 2 selected from the group consisting of: linear or branched C 1 -C 8 Alkyl, phenyl, saturated monocyclic C 3 -C 8 Cycloalkyl, saturated bicyclic C 4 -C 10 Cycloalkyl, saturated tricyclic C 7 -C 10 Cycloalkyl, oxetanyl, tetrahydrofuranyl and tetrahydropyranyl; or
[0039] R 2 is selected from the group con...
example 1
[0175] Intermediate 1:
[0176]
[0177] 3-Propyl-[1,2,4]triazolo[4,3-a]pyrazin-8(7H)-one:
[0178] To a solution of commercially available 2-chloro-3-hydrazinopyrazine (28 g, 0.19 mol) in dichloromethane (200 mL) was added butyraldehyde (14.7 g, 0.20 mol) and the reaction was allowed to cool before cooling to 0 °C. Stir at 50°C for 2 hours. Diacetoxyiodobenzene (71.8 g, 0.223 mol) was added at 0°C. The cooling was removed and the mixture was stirred at 20 °C for 2 hours. Na 2 CO 3 (80 mL) of saturated aqueous solution was slowly poured into the reaction, and the mixture was stirred for 10 min. The organic phase was separated, washed with brine (3×50 mL), washed with Na 2 SO 4 Dry and concentrate under vacuum. The residue was purified by flash chromatography on silica gel using a gradient of ethyl acetate and petroleum ether to give 8-chloro-3-propyl-[1,2,4]triazolo[4,3-a]pyridine Azine 20 g (53%).
[0179] Dioxane (120mL) and H 2 A mixture of 8-chloro-3-propyl-[...
example 2
[0181]
[0182] 3-Propyl-7-((tetrahydro-2H-pyran-3-yl)methyl)-[1,2,4]triazolo[4,3-a]pyrazine-8(7H)- ketone:
[0183] Step 1: To a solution of (tetrahydro-2H-pyran-3-yl)methanol (300 mg, 2.58 mmol) and TEA (522 mg, 5.17 mmol) in dichloromethane (10 mL) was added methanesulfonate at 0 °C Acid chloride (355 mg, 3.10 mmol) and the reaction was allowed to warm to 20°C and stir for 1 hour. The solution was washed with saturated NaHCO 3 Aqueous solution (2mL), H 2 O (3 x 2 mL), washed with brine (1 mL), dried and concentrated to give (tetrahydro-2H-pyran-3-yl)methyl mesylate (500 mg), which was used directly in the next step .
[0184] Step 2: Dissolve (tetrahydro-2H-pyran-3-yl)methyl mesylate (500 mg, 2.58 mmol) and 3-propyl-[1,2,4]triazole in DMF (30 mL) And[4,3-a]pyrazin-8(7H)-one (413mg, 2.32mmol) was added K 2 CO 3 (534 mg, 3.87 mmol). The mixture was stirred at 60°C for 4 hours. The reaction was cooled to RT and diluted with DCM (100 mL). Wash the organic phase wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com