A method for synthesizing 1-difluoromethylimidazole and derivatives thereof
A technology of difluoromethylimidazole and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high price and ozone depletion, and achieve the effects of low price, low environmental hazards and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add benzimidazole (0.5mmol), ethyl difluorobromoacetate (0.6mmol), potassium hydroxide (0.5mmol), and acetonitrile (1ml) to a 25mL pressure-proof bottle in turn, seal the pressure-proof bottle, and put it into the pre- Heated to 60 ° C in an oil bath for 6h. After the reaction, cool to room temperature. 30 mL of water was added to the reaction solution, and extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product. The crude product was subjected to column chromatography using a mixed solvent of petroleum ether and ethyl acetate as the eluent to obtain the target compound with a yield of 94%.
Embodiment 2
[0020] Add 5,6-dimethylbenzimidazole (0.5mmol), ethyl difluorobromoacetate (0.6mmol), potassium hydroxide (0.5mmol), and acetonitrile (1ml) to a 25mL pressure-resistant bottle in sequence, and seal the pressure-resistant bottle, and put it into an oil bath preheated to 60°C to react for 6h. After the reaction, cool to room temperature. 30 mL of water was added to the reaction solution, and extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product. The crude product was subjected to column chromatography using a mixed solvent of petroleum ether and ethyl acetate as the eluent to obtain the target compound with a yield of 87%.
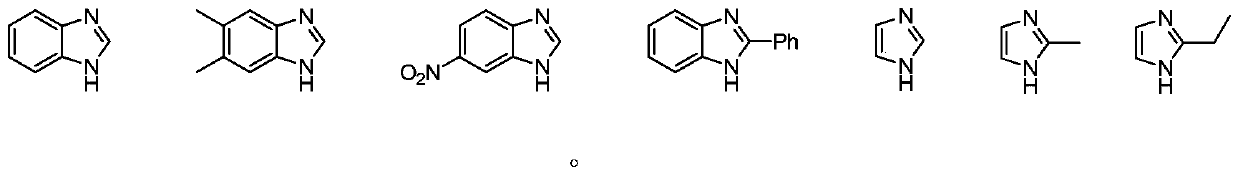
[0021] The benzimidazole was replaced by 5-nitrobenzimidazole, and the yield was 79%. The benzimidazole was replaced by 2-phenylbenzimidazole, and the yield was 93%. The benzimidazole was replaced by imidazole, and the yield was 76%. The benzimidazole...
Embodiment 3
[0023] Reaction steps are identical with embodiment 1, difference is:
[0024] The amount of potassium hydroxide used was 0.75 mmol, and the yield of 1-difluoromethylbenzimidazole was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com