Method for preparing high-content nemadectin
A technology of protecting group and oxo generation, which is applied in the field of preparing high-content nemoctine, and can solve the problem of low content of nemoctine products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The technical process of present embodiment preparation high-content nemoctine is as follows:
[0034]
[0035] (1) Reduction reaction:
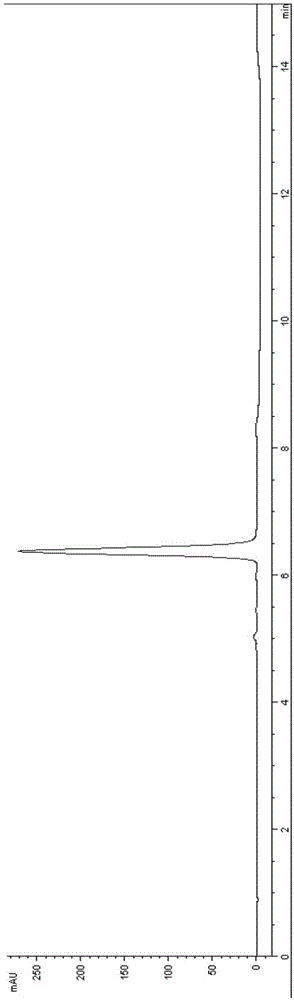
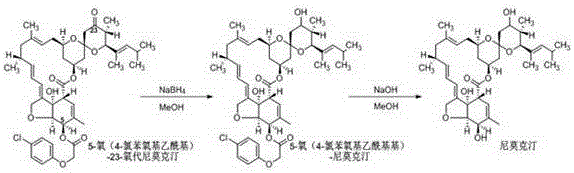
[0036] Add 4.0g of 5-oxo(4-nitrobenzoyl)-23-oxonimoxetine (5.0mmol, 1.0eq, HPLC purity 95.0A%, white powder) into a 100mL three-necked flask, add 40.0g of methanol and stir After uniformity, a milky white suspension was obtained, put it into an ice-water bath and cool to 0~5°C, add 567mg (15.0mmol, 3.0eq) of sodium borohydride in batches, keep stirring for 1.0h after the addition, and pour the reaction solution into 40.0g of water , extracted twice with 40.0 g of dichloroethane, and the obtained oil layer was evaporated under negative pressure to remove the solvent to obtain 4.0 g (4.9 mmol, HPLC purity 94.0A%).
[0037] (2) Deprotection reaction:
[0038] 4.0g (4.9mmol, 1.0eq) of 5-oxo(4-nitrobenzoyl)-nimoctine obtained in step (1) was mixed with dichloroethane (20.0g) and methanol (20.0g) After dissolving, transfer it to a 10...
Embodiment 2
[0042]The technical process of present embodiment preparation high-content nemoctine is as follows:
[0043]
[0044] (1) Reduction reaction:
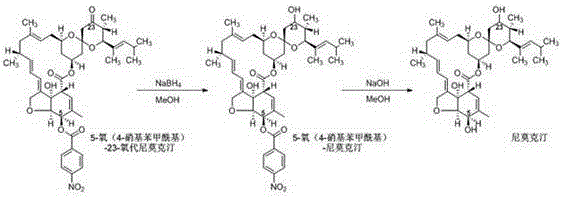
[0045] Add 8.1g of 5-oxo(4-chlorophenoxyacetyl)-23-oxonimoxetine (10.0mmol, 1.0eq, HPLC purity 96.2A%, white powder) into a 250mL three-necked flask, add methanol 81.0g Stir evenly to obtain a milky white suspension, put it in an ice-water bath to cool to 0~5°C, add 1135mg (30.0mmol, 3.0eq) of sodium borohydride in batches, keep stirring for 1.0h after the addition, and pour the reaction solution into 80.0g In water, extracted twice with 80.0 g of dichloroethane, and the obtained oil layer was vacuum-rotated to remove the solvent to obtain light yellow 5-oxo(4-chlorophenoxyacetyl)-nimoctine 8.0 g (9.8 mmol, HPLC purity 96.0A%).
[0046] (2) Deprotection reaction:
[0047] 8.0g (9.8mmol, 1.0eq) of the above-mentioned 5-oxo(4-chlorophenoxyacetyl)-Nimoctine was dissolved in 40.0g dichloroethane and 40.0g methanol, then transferred t...
Embodiment 3
[0051] The technical process of present embodiment preparation high-content nemoctine is as follows:
[0052]
[0053] (1) Deoxime reaction:
[0054] Add 4.0g (4.9mmol, 1.0eq, HPLC purity 97.02A%, white powder) of 5-oxo(4-nitrobenzoyl)-23-methoximoxine to a 100mL three-necked flask, add dioxane 40.0g dissolved clear, then added 3.6g hydrochloric acid (9.9g, 97.6mmol, 20.0eq, content 36wt.%), then heated to 50°C and kept stirring for 10.0h. The reaction solution was poured into 40.0g of water, extracted twice with 40.0g of dichloroethane, and the obtained oil layer was rotated to dryness under negative pressure to obtain light yellow 5-oxo(protecting group)-23-oxonimoxetin 3.8g (4.8mmol, HPLC purity 96.5A%, yield 98.0%).
[0055] (2) The reduction reaction, deprotection reaction and silica gel column separation process were the same as in Example 1, and finally 2.8 g of nimoctine (HPLC purity 96.5A%, content 93.7 wt.%, white solid) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com