Method for determining content and impurities of levocarnitine injection and use thereof
A technology for injection and impurities, applied in the determination of levocarnitine injection content and impurities, hydrophilic interaction chromatography determination of levocarnitine injection content and impurities, can solve the requirements of low separation limit and theoretical plate number Reduced, short life of the column and other problems, to achieve the effect of overcoming the hard-to-obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
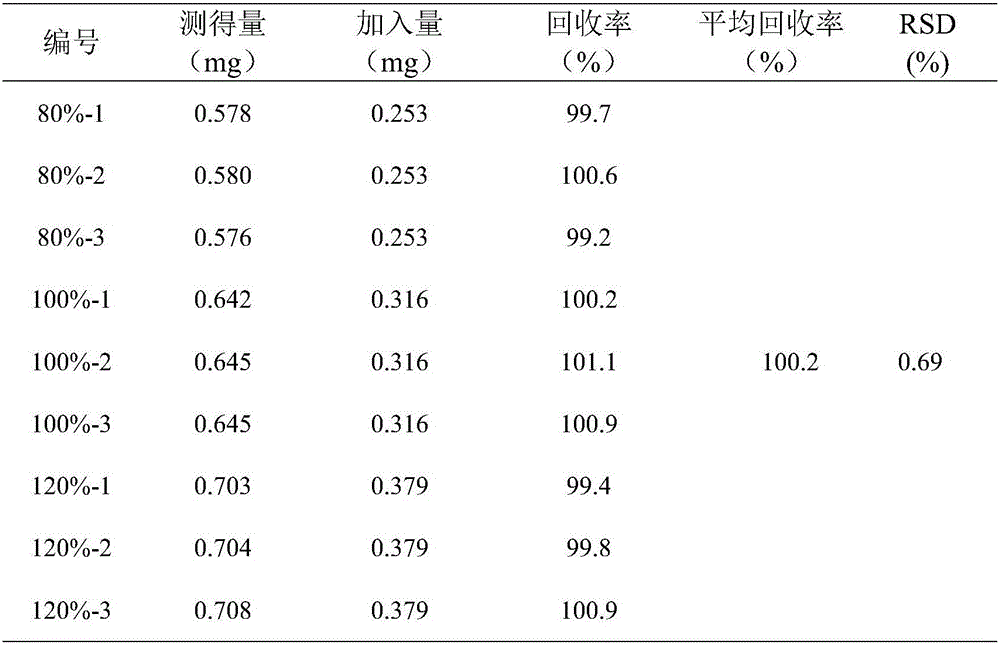
[0033] The following examples help to further understand the present invention, but the present invention is not limited to these contents.
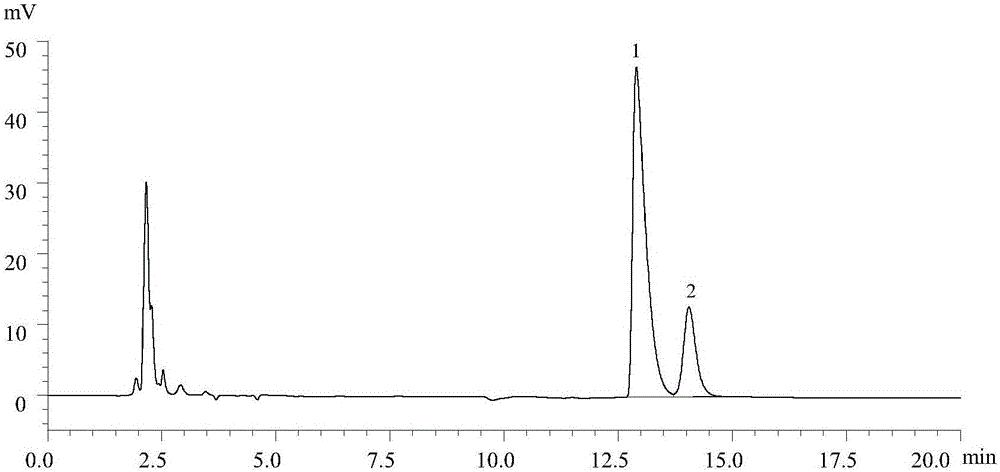
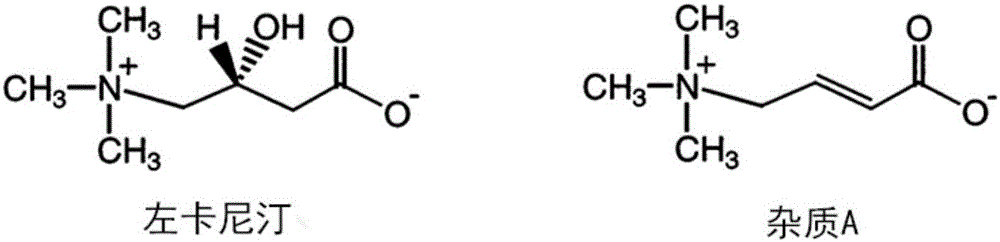
[0034] A method for determining the content and impurities of levocarnitine injection by hydrophilic interaction chromatography.
[0035] 1 material preparation
[0036] Shimadzu LC-20A high performance liquid chromatography system (including LC-20AT pump; DGU-20A degasser; SIL-20A autosampler; CTO-10ASvp column thermostat; SPD-20A UV detector; Lab solution chromatography Workstation); WatersXBridge Amide chromatographic column (4.6mm × 250mm, 3.5μm); 1 / 10,000 electronic balance (AR1140, OhausCorp.Brook, NJ, USA); 1 / 100,000 electronic balance (MS105Du type, METTLER TOLEDO); Electronic pH meter (pHMeter PHS-3C, Shanghai Jingke Industrial Co., Ltd.); ultrasonic cleaner (SK250HP, Wuxi Jianyi Experimental Equipment Co., Ltd.).
[0037] The reference substance of levocarnitine was provided by China National Institutes for Food and Drug Cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com