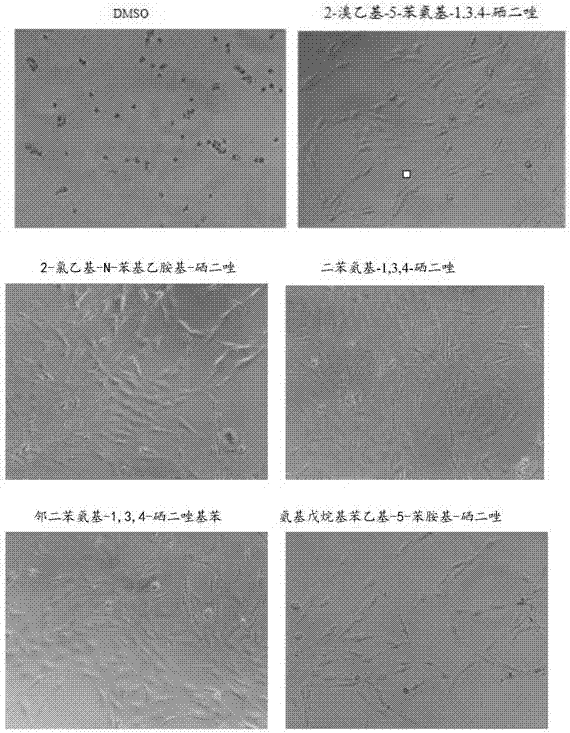
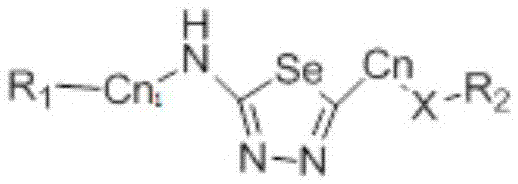
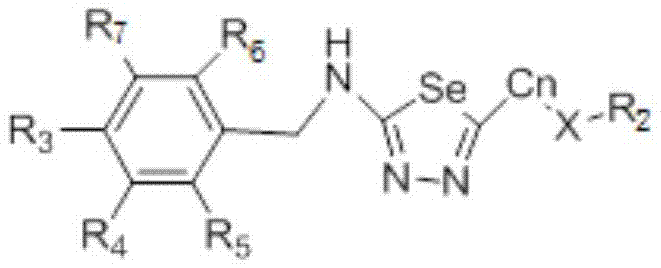
1,3,4-selenadiazole compound with drug activity
A kind of technology of medicinal activity and selenium diazoles, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0059] The preparation method process takes phenylselenourea as an example and enumerates as follows:
[0060] Phenylselenourea (1mmol; or other selenoureas), compounds with carboxylic acid groups (0.2-4mmol), and 5mlPOCl 3Mix well and stir well. Raise the temperature to 50-80°C, react for 0.5-12h, stop heating in the oil bath, drain the reaction solution under reduced pressure, slowly pour into ice water, filter with suction to obtain a filter cake, separate by column chromatography, and dry. The product was obtained with a yield of 40-95%.
[0061] 2-Bromoethyl-N-phenylamino-1,3,4-selenoadiazole:
[0062]
[0063] m / z 332 (100%, M+H + )
[0064] 1 H NMR (500MHz,) δ10.37(s, 1H), 7.65–7.53(m, 2H), 7.35(d, J=9.0Hz, 2H), 6.99(dt, J=7.4, 3.7Hz, 1H), 3.6(t, J=6.5Hz, 2H), 3.38-3.29(t, J=6.5Hz, 2H)
[0065] 2-Bromoethyl-N-phenylamino-1,3,4-selenoadiazole:
[0066]
[0067] Substituted selenourea (1mmol; or other selenourea), and multifunctional carboxylic acid compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com