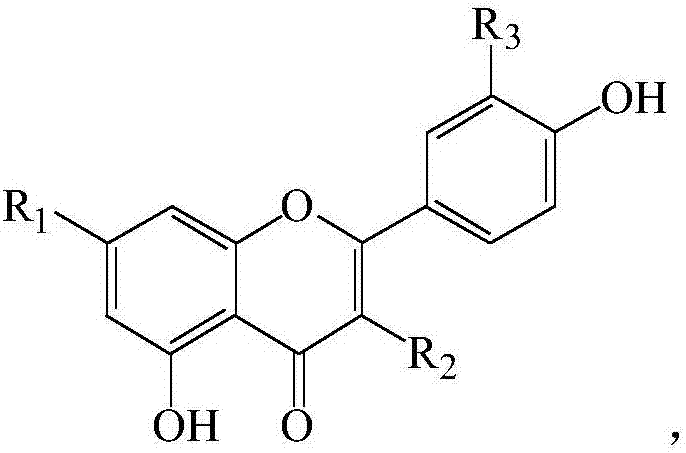
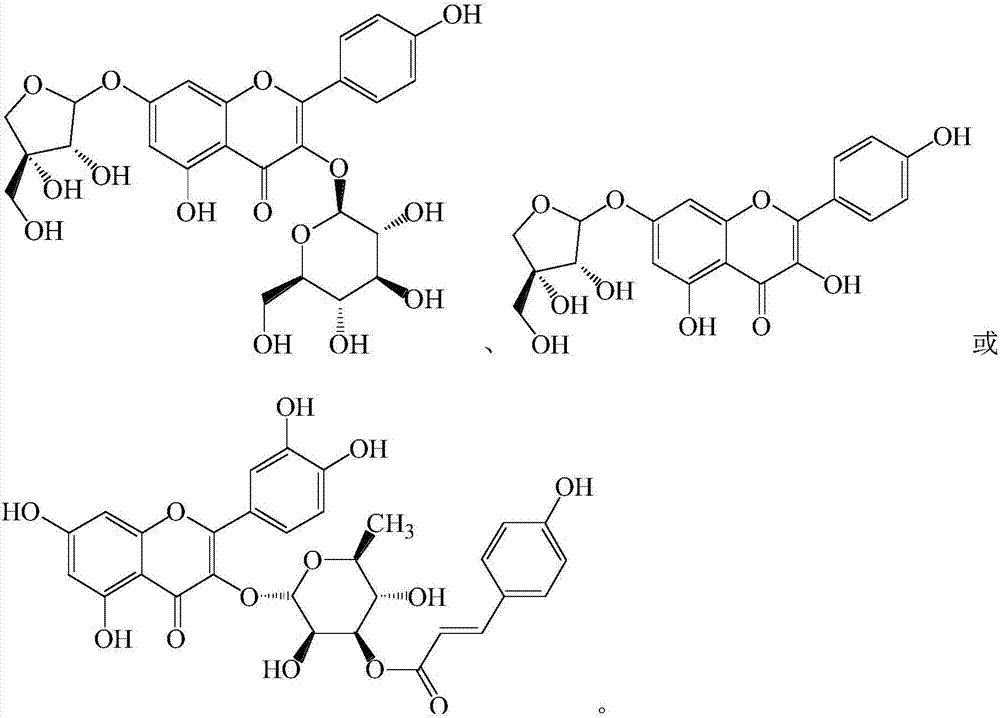
3,5,7-trihydroxy-2-(4-hydroxyphenyl)-benzo pyranyl-4-one derivatives
A technology of hydroxyphenyl and benzopyran is applied in the field of 3,5,7-trihydroxy-2--benzopyran-4-one derivatives, which can solve problems such as seed residue and achieve good anti-oxidation The effect of action, stable structure and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0019] 1) Preliminary extraction:
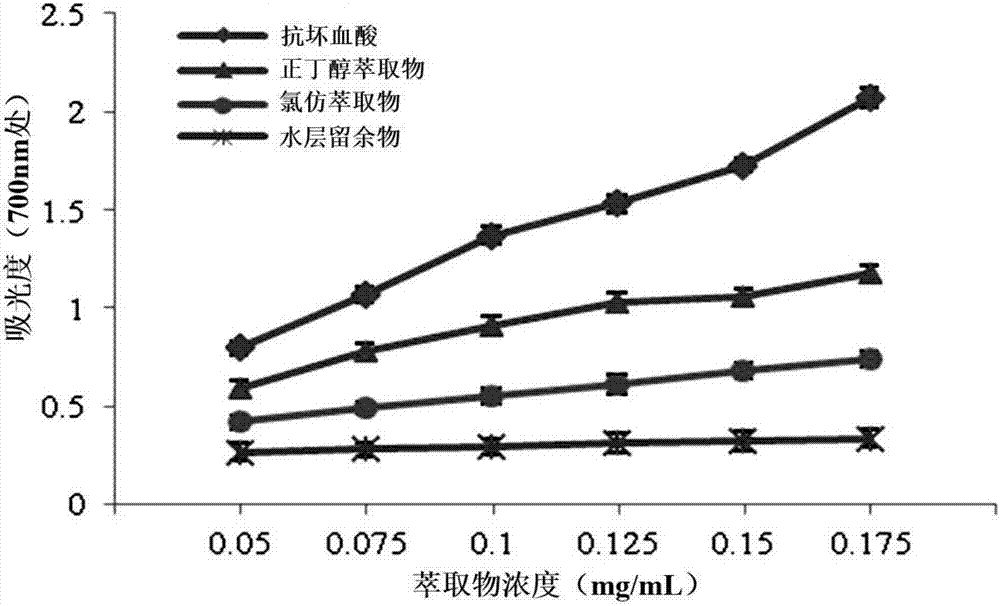
[0020] Grind 15kg of dry seeds of Desmodium sativa, degrease with petroleum ether, then reflux and extract with 95% ethanol for 3 times, the time of each reflux is 3h, combine the extracts, concentrate under reduced pressure to obtain an extract, add appropriate amount of distilled water to suspend, Then extract with chloroform and water-saturated n-butanol for several times in sequence, and concentrate each part of the extract under reduced pressure to obtain 171 g of chloroform extract, 664 g of n-butanol extract and 893 g of the residue in the water layer.
[0021] 2) Determination of total flavonoid content:
[0022] ① Preparation of test sample solution
[0023] Weigh 63.5mg of chloroform extract, 45.2mg of n-butanol extract, and 175mg of the residue in the water layer, respectively, and place them in three 25mL volumetric flasks, dissolve and dilute to the mark with 70% ethanol, shake well, and refrigerate at 4°C ,stand-by.
[0024]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com