Cold storage gel, preparation method thereof and cold application patch
A technology of cold gel and konjac gum powder is applied in the field of medical supplies, which can solve the problems of inconvenient wearing, poor gel fit, and no antibacterial barrier.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
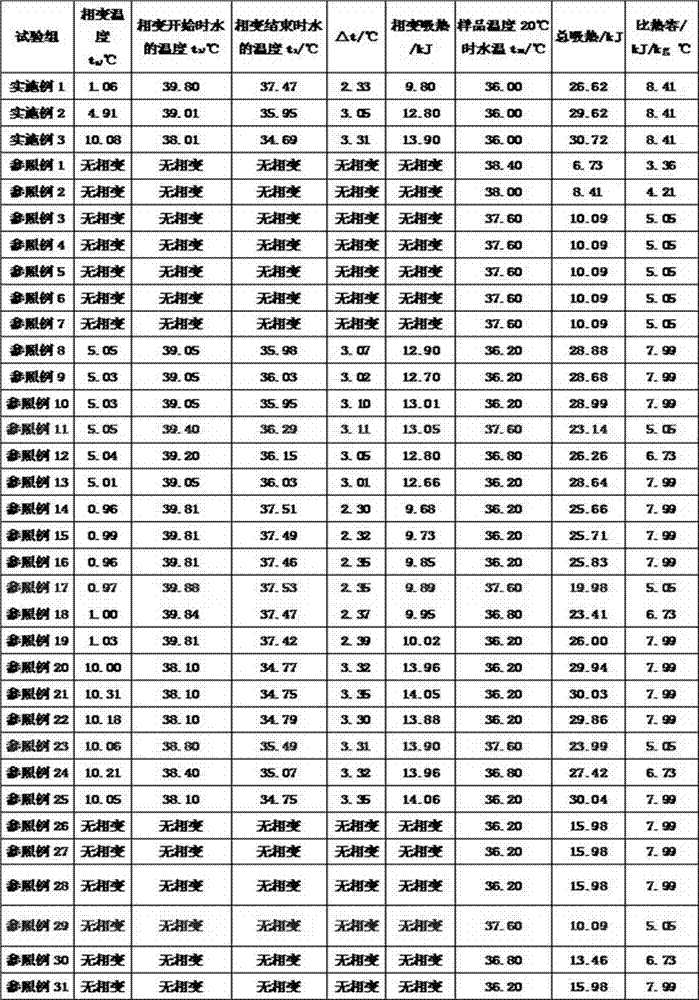
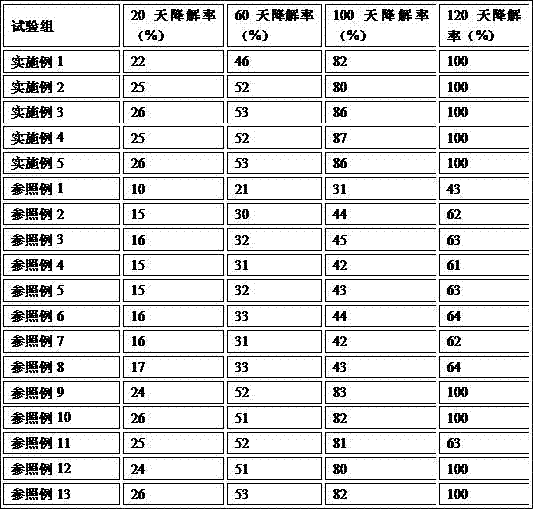
[0025] (1) Preparation of solution A: 0.3% by weight of menthol, 0.5% of vitamin E, 19% of glycerin, and 0.4% of apple extract were added to ethanol with a weight percentage of 4% as a solvent;
[0026] (2) Prepare solution B: add 0.8% hemicellulose and 0.2% konjac gum powder to 74.7% by weight purified water, and dissolve at 50°C until completely dissolved;
[0027] (3) Prepare solution C: accurately weigh 100g of potato starch and 3g of sodium trimetaphosphate in a test tube, add 400ml of purified water, and slowly add a 10% NaOH solution dropwise while stirring at 40 degrees Celsius. Keep the reaction pH value at 11, and keep it warm for 3 hours to obtain a cross-linked potato starch solution;
[0028] (4) Stir the 0.1% by weight solution C with the above solutions A and B at 40°C to form a gel.
Embodiment 2
[0030] (1) Preparation of solution A: 0.3% by weight of menthol, 0.5% of vitamin E, 19% of glycerin, and 0.4% of apple extract were added to ethanol with a weight percentage of 4% as a solvent;
[0031] (2) Preparation of solution B: Add 0.8% hemicellulose and 0.2% konjac gum powder to 74.3% by weight purified water, until completely dissolved at 50°C;
[0032] (3) Prepare solution C: accurately weigh 100g of potato starch and 3g of sodium trimetaphosphate in a test tube, add 400ml of purified water, and slowly add a 10% NaOH solution dropwise while stirring at 40 degrees Celsius. Keep the reaction pH value at 11, and keep it warm for 3 hours to obtain a cross-linked potato starch solution;
[0033] (4) Stir the 0.5% by weight solution C with the above solutions A and B at 40°C to form a gel.
Embodiment 3
[0035] (1) Preparation of solution A: 0.3% by weight of menthol, 0.5% of vitamin E, 19% of glycerin, and 0.4% of apple extract were added to ethanol with a weight percentage of 4% as a solvent;
[0036] (2) Preparation of solution B: Add 0.8% hemicellulose and 0.2% konjac gum powder to 72.8% by weight purified water, until completely dissolved at 50°C;
[0037] (3) Prepare solution C: accurately weigh 100g of potato starch and 3g of sodium trimetaphosphate in a test tube, add 400ml of purified water, and slowly add a 10% NaOH solution dropwise while stirring at 40 degrees Celsius. Keep the reaction pH value at 11, and keep it warm for 3 hours to obtain a cross-linked potato starch solution;
[0038] (4) Stir the 2% by weight solution C with the above solutions A and B at 40°C to form a gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com