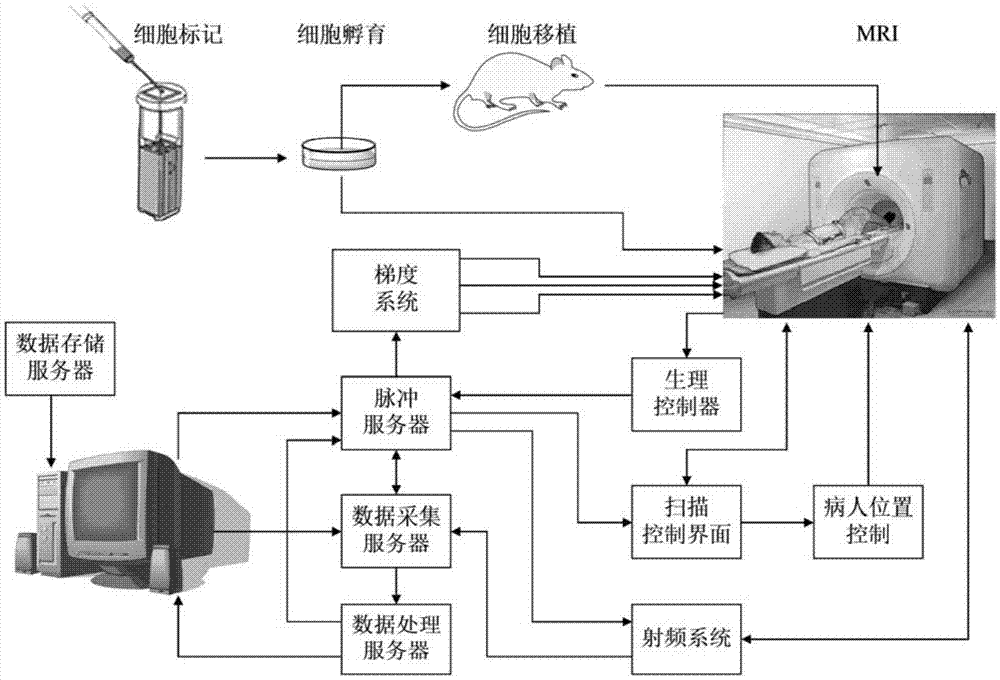
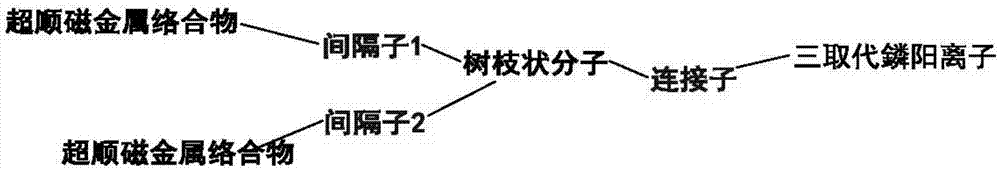
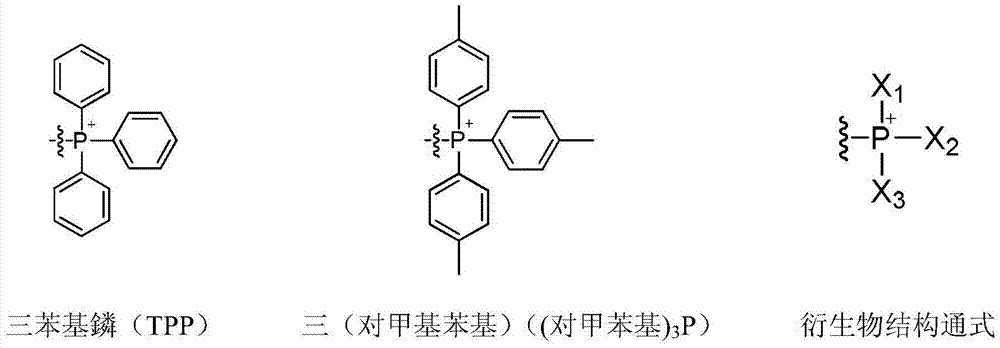
Application of mitochondrial targeting contrast media molecule as T2 contrast media
A contrast agent and mitochondrial technology, applied in the field of medical imaging, can solve the problems that the duration of the signal enhancement effect cannot meet the long-term observation, and the survival and migration status of labeled cells cannot be clearly determined.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Synthesis of the contrast agent molecule Gd-DOTA-TPP targeting mitochondria ( Image 6 )
[0072] 1. Bu t 3 Synthesis of DOTA (1,4,7-tris(tert-butoxycarbonylmethyl)-10-(acetic acid)-1,4,7,10-tetraazacyclododecane). Synthesize starting from 1,4,7,10-tetraazacyclododecane (Cyclen) as follows:
[0073] a) Weigh 10.0g Cyclen and 29.3g NaHCO 3 Place in a 1L three-necked flask, and add 50mL of acetonitrile. Weigh 37.4g of tert-butyl bromoacetate in a fume hood, add 20mL of acetonitrile, mix well, and put it into the dropping funnel. in an ice bath and N 2 Under protection, the acetonitrile solution of tert-butyl bromoacetate was slowly added dropwise to the reaction mixture in the three-neck flask. After the dropwise addition was completed, stirring was continued at room temperature for 30 hours. The solid was filtered off, acetonitrile was removed by rotary evaporation, and recrystallized twice with toluene to obtain 16 g of white solid Bu t 3 DO3A (1,4,...
Embodiment 2
[0083] Embodiment 2: (Gd-DOTA) 4 Synthesis of -TPP-targeted mitochondrial contrast agent molecules ( Figure 7 )
[0084] 1, synthesize Bu according to embodiment 1 t 3 DOTA and Ph 3 P(Br)(CH 2 ) 4 COOH.
[0085] 2. Synthesize DOTA by solid phase synthesis 4 -TPP: The steps are briefly described as follows: Synthesize on a solid-phase synthesizer according to the traditional Fmoc method, press Figure 7 In the structure shown, amino acids are sequentially coupled from the C-terminal to the N-terminal. First solid phase carrier 2-chlorotrityl resin 1g, add 2.0g Fmoc-Lys(Mtt)-OH successively, 1.77gPh 3 P(Br)(CH 2 ) 4 COOH is condensed, and the conditions for the condensation of carboxyl and amino groups in each step are 50mL of DMF as a solvent, adding 0.96g of TBTU, 0.41g of HOBt condensing agent and 2.5mL of base DIPEA, and reacting at 25°C for about 24 hours. Ketone color development shall prevail to determine whether the condensation of carboxyl and amino groups ...
Embodiment 3
[0090] Embodiment 3: (Gd-DOTA) 4 -linker-TPP synthesis of contrast agent molecules targeting mitochondria (the spacer here is Acp) ( Figure 8 , dendrimers)
[0091] 1. Synthesize Bu according to the method of Example 1 t 3 DOTA and Ph 3 P(Br)(CH 2 ) 4 COOH.
[0092] 2. Synthesize DOTA by solid phase synthesis 4 -linker-TPP:
[0093] The steps are briefly described as follows: synthesize on a solid-phase synthesizer by the traditional Fmoc method, press Figure 8 In the structure shown, amino acids are sequentially coupled from the C-terminal to the N-terminal. First, solid phase carrier 2-chlorotrityl resin 1g, add 2.0g Fmoc-Lys(Mtt)-OH, 1.77g Ph 3 P(Br)(CH 2 ) 4 COOH is condensed, and the conditions for the condensation of carboxyl and amino groups in each step are 50mL of DMF as a solvent, adding 0.96g of TBTU, 0.41g of HOBt condensing agent and 2.5mL of base DIPEA, and reacting at 25°C for about 24 hours. Ketone color development shall prevail to determine whe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com