Method for recycling oxalic acid and hydrochloric acid from rare earth oxalate precipitation wastewater
A technology of oxalic acid precipitation and oxalic acid, applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, water pollutants, etc., can solve problems such as increased production costs, unfavorable stripping, and increased difficulty in cleaning precipitation, and achieves reduction The effect of small volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
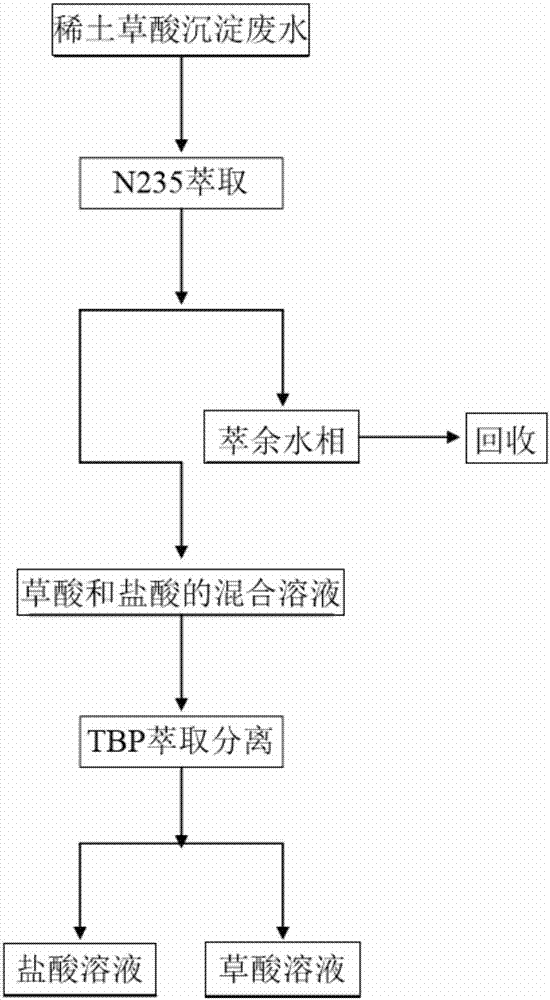
[0031] In the present embodiment, the method for reclaiming oxalic acid and hydrochloric acid from rare earth oxalic acid precipitation wastewater comprises the following steps:
[0032] 1. Mix N235 with sulfonated kerosene and octanol to obtain an extractant whose volume fraction is 15% of N235, 65% by volume of sulfonated kerosene, and 20% by volume fraction of octanol.
[0033] 2. Mix the rare earth oxalic acid precipitation wastewater with the extractant at a volume ratio of 5:1, the extraction temperature is 25°C, and after 10 stages of extraction, an organic phase loaded with oxalic acid and hydrochloric acid is obtained, and the extraction rates of oxalic acid and hydrochloric acid reach 98%, respectively. 99%.
[0034] 3. Mix the organic phase loaded with oxalic acid and hydrochloric acid and deionized water in a volume ratio of 3:1, and the stripping temperature is 55°C. After 15 stages of stripping, a mixed solution of enriched oxalic acid and hydrochloric acid is ob...
Embodiment 2
[0039] In the present embodiment, the method for reclaiming oxalic acid and hydrochloric acid from rare earth oxalic acid precipitation wastewater comprises the following steps:
[0040] 1. Mix N235 with sulfonated kerosene and octanol to obtain an extractant with a volume fraction of N235 of 20%, a volume fraction of sulfonated kerosene of 65%, and an octanol volume fraction of 15%.
[0041] 2. Mix the rare earth oxalic acid precipitation wastewater with the extractant at a volume ratio of 1:1. The extraction temperature is 35°C. After 12 stages of extraction, an organic phase loaded with oxalic acid and hydrochloric acid is obtained. The extraction rates of oxalic acid and hydrochloric acid reach 98%, respectively. 99%.
[0042] 3. Mix the organic phase loaded with oxalic acid and hydrochloric acid and deionized water in a volume ratio of 5:1, and the stripping temperature is 45°C. After 10 stages of stripping, a mixed solution of enriched oxalic acid and hydrochloric acid is ...
Embodiment 3
[0047] In the present embodiment, the method for reclaiming oxalic acid and hydrochloric acid from rare earth oxalic acid precipitation wastewater comprises the following steps:
[0048] 1. N235 is mixed with sulfonated kerosene and octanol to obtain an extractant with a volume fraction of N235 of 25%, a volume fraction of sulfonated kerosene of 65%, and an octanol volume fraction of 10%.
[0049] 2. Mix the rare earth oxalic acid precipitation wastewater with the extractant at a volume ratio of 0.5︰1. The extraction temperature is 45°C. After 15 stages of extraction, the organic phase loaded with oxalic acid and hydrochloric acid is obtained. The extraction rates of oxalic acid and hydrochloric acid reach 98%, respectively. 99%.
[0050] 3. Mix the organic phase loaded with oxalic acid and hydrochloric acid and deionized water in a volume ratio of 7:1, and the stripping temperature is 35°C. After 8 stages of stripping, a mixed solution of enriched oxalic acid and hydrochloric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com