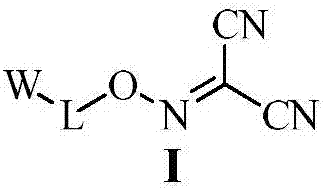
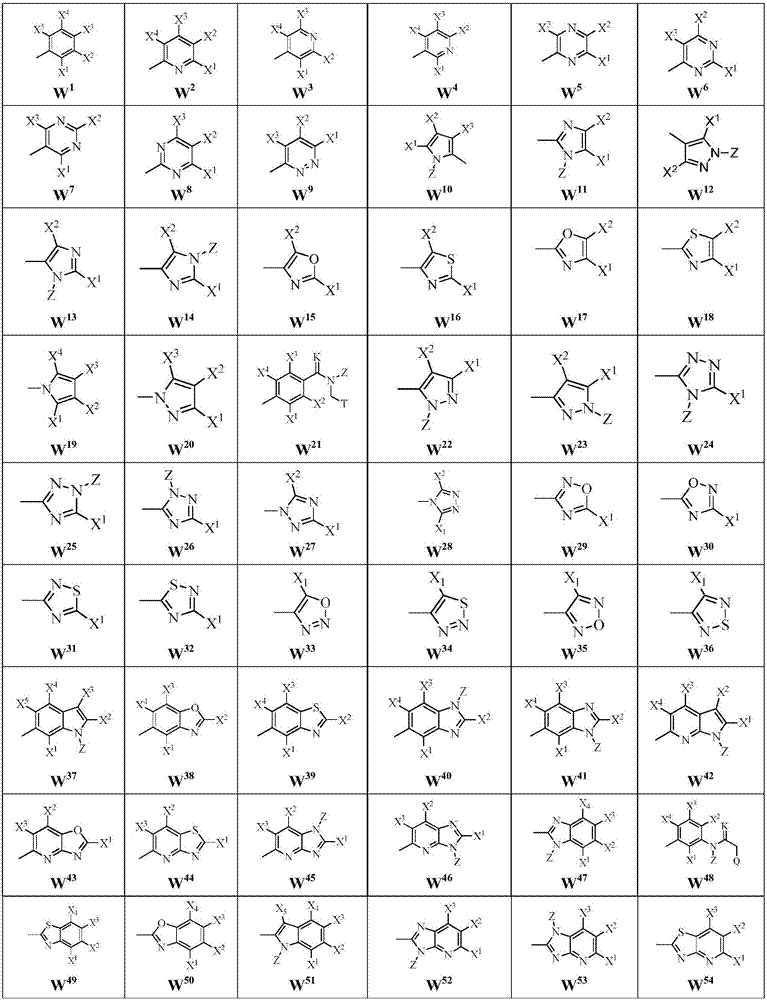
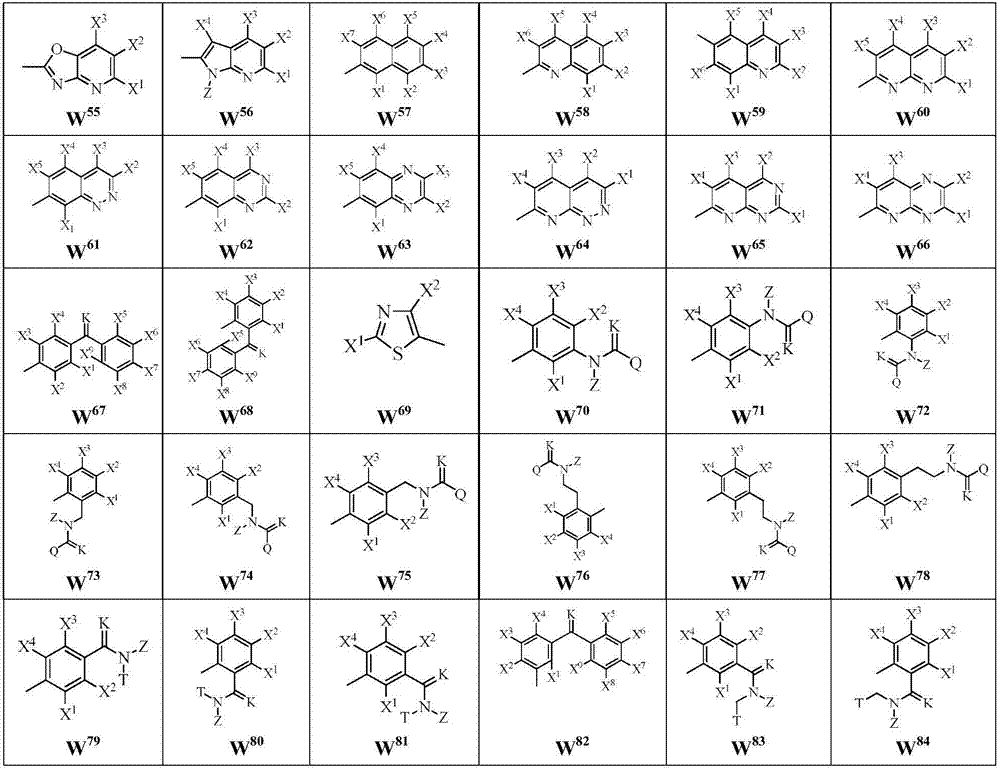
Malononitrile oxime ether compound and use thereof
A technology of ether compounds and malononitrile oxime, which is applied in the fields of application, organic chemistry, and biocides, and can solve the problems that the bactericidal activity of malononitrile oxime ether compounds has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Embodiment 1: the preparation of compound 4
[0201]
[0202] Add p-chlorobenzyl chloride (0.3 g, 1.86 mmol), malononitrile oxime ether sodium salt (0.22 g, 1.86 mmol) and 15 ml of acetonitrile in a 50 ml reaction flask, stir the reaction at room temperature, TLC (ethyl acetate: Petroleum ether=1:8) monitoring, after the completion of the reaction, acetonitrile was evaporated under reduced pressure, and the residue column chromatography (ethyl acetate:petroleum ether=1:10 was used as eluent, 100 -140 mesh silica gel) to obtain 0.21 g of yellow oil, with a yield of 51%.
Embodiment 2
[0203] Embodiment 2: the preparation of compound 25
[0204]
[0205] Add p-trifluoromethoxybenzyl bromide (0.25 gram, 0.98 mmol), malononitrile oxime ether sodium salt (0.12 gram, 0.98 mmol) and 15 milliliters of acetonitrile in 50 milliliters of reaction flasks, stir reaction at room temperature, TLC ( Ethyl acetate:petroleum ether=1:8) monitoring, after the completion of the reaction, acetonitrile was evaporated under reduced pressure, and the residue column chromatography (ethyl acetate:petroleum ether=1:10 was used as eluent, Qingdao Marine Biochemical Factory 100-140 mesh silica gel produced in the factory) was purified to obtain 0.16 g of white solid, with a yield of 61%.
Embodiment 3
[0206] Embodiment 3: the preparation of compound 37
[0207]
[0208] Add p-cyanobenzyl bromide (0.3 g, 1.53 mmol), malononitrile oxime ether sodium salt (0.18 g, 1.53 mmol) and 15 ml of acetonitrile in a 50 ml reaction flask, stir the reaction at room temperature, TLC (ethyl acetate : Petroleum ether=1:8) monitoring, after the completion of the reaction, acetonitrile was evaporated under reduced pressure, and the residue column chromatography (ethyl acetate: petroleum ether=1:10 was used as eluent, produced by Qingdao Marine Biochemical Factory Branch Factory 100-140 mesh silica gel) was purified to obtain 0.18 g of white solid with a yield of 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com