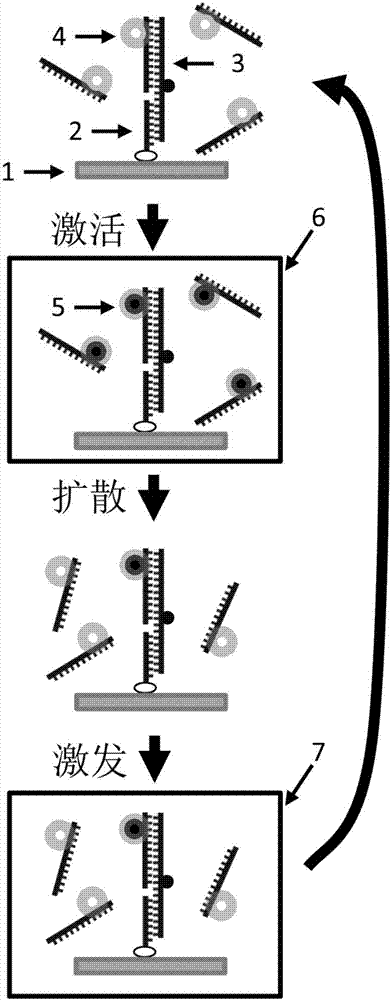
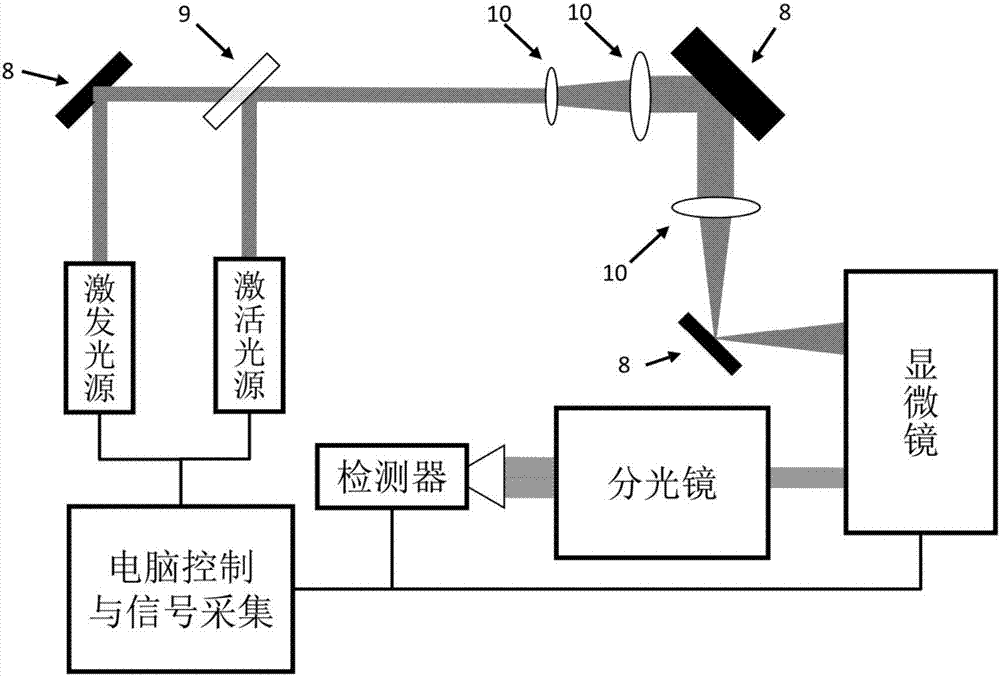
Monomolecular fluorescence resonance energy transfer method based on photo-activation
A technology of fluorescence resonance energy and light activation, which is applied in the fields of fluorescence/phosphorescence, material excitation analysis, material analysis by optical means, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1, Single-molecule fluorescence resonance energy transfer based on CAGE 552 photoactivatable dye.
[0035] Using the nucleic acid molecule labeled with CAGE 552 light-activated dye as a model, the observation of single-molecule fluorescence resonance energy transfer at a sample concentration of micromolar is realized.
[0036] The specific experiment is as follows:
[0037]1 mg of N-hydroxysuccinimide ester (NHS ester)-activated CAGE 552 photoactivatable dye was purchased from Abberior and dissolved in 181 μL of dimethyl sulfoxide so that the final concentration of the dye was 10 mM. CAGE 552 dye can be activated by 405nm laser and excited by 532nm laser. Purchase 1 mg of N-hydroxysuccinimide ester (NHSester)-activated Cyanine5 non-photoactivatable dye from Lumiprobe Company, and dissolve it in 162 μL of dimethyl sulfoxide, so that the final concentration of the dye is 10 mM. The biological sample is nucleic acid (DNA). A 100 μL 50 μM DNA single-stranded molec...
Embodiment 2
[0038] Example 2, single-molecule fluorescence resonance energy transfer based on CAGE FAM light-activated dye.
[0039] Using the nucleic acid molecule labeled with CAGE FAM light-activated dye as a model, the observation of single-molecule fluorescence resonance energy transfer at the sample concentration at the micromolar level is realized.
[0040] The specific experiment is as follows:
[0041] Purchase 1 mg of N-hydroxysuccinimide ester (NHS ester) activated CAGE FAM (CMNB-cagedcarboxyfluorescein, SE) photoactivatable dye from Invitroge, dissolve it in 103 μL of dimethyl sulfoxide, so that the final concentration of the dye is 10 mM . CAGE FAM dyes can be activated by 405nm laser and excited by 488nm laser. Purchase 1 mg of N-hydroxysuccinimide ester (NHS ester)-activated Cyanine5 non-photoactivatable dye from Lumiprobe, and dissolve it in 162 μL of dimethyl sulfoxide so that the final concentration of the dye is 10 mM. The biological sample is nucleic acid (DNA). A 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com