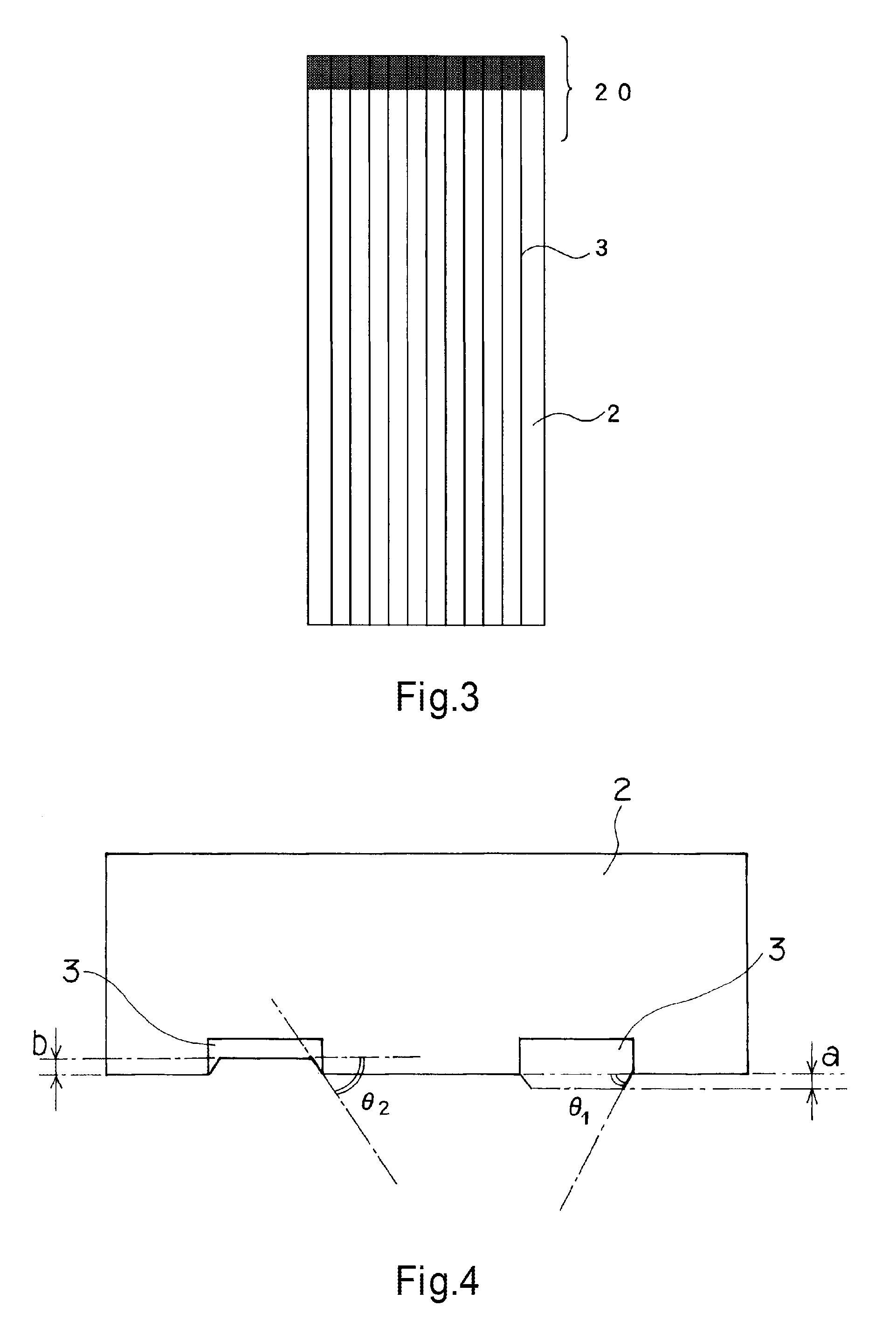
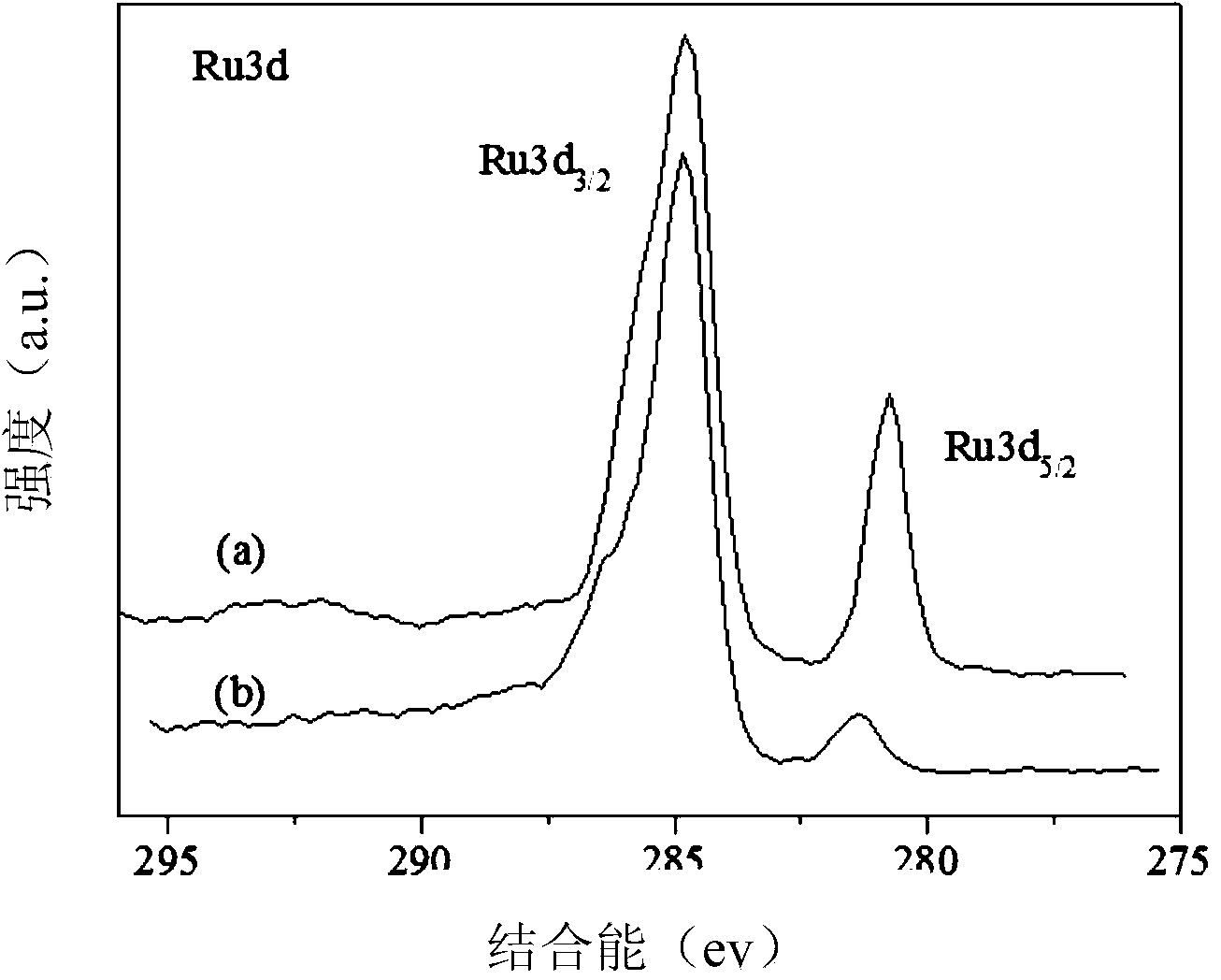
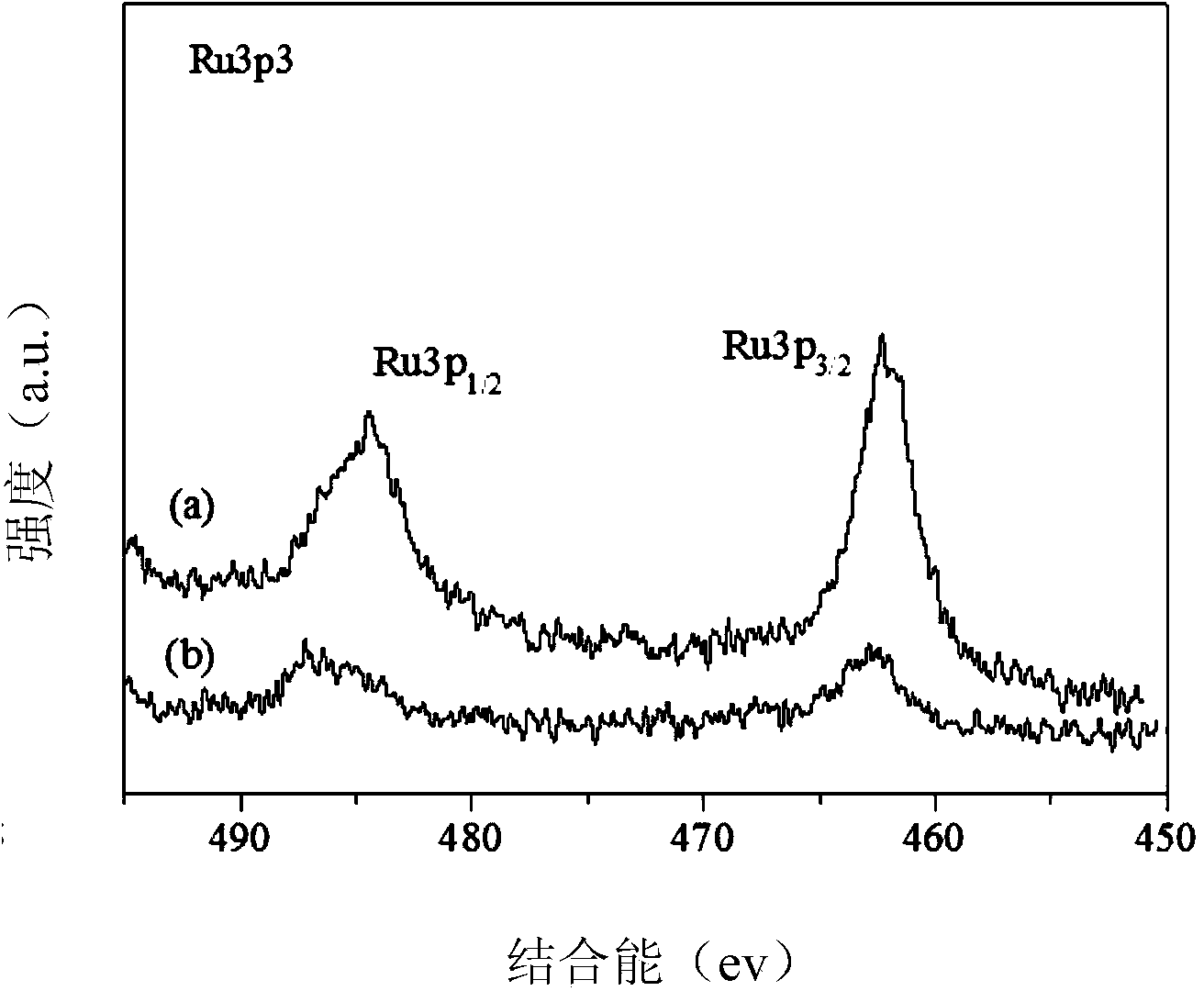
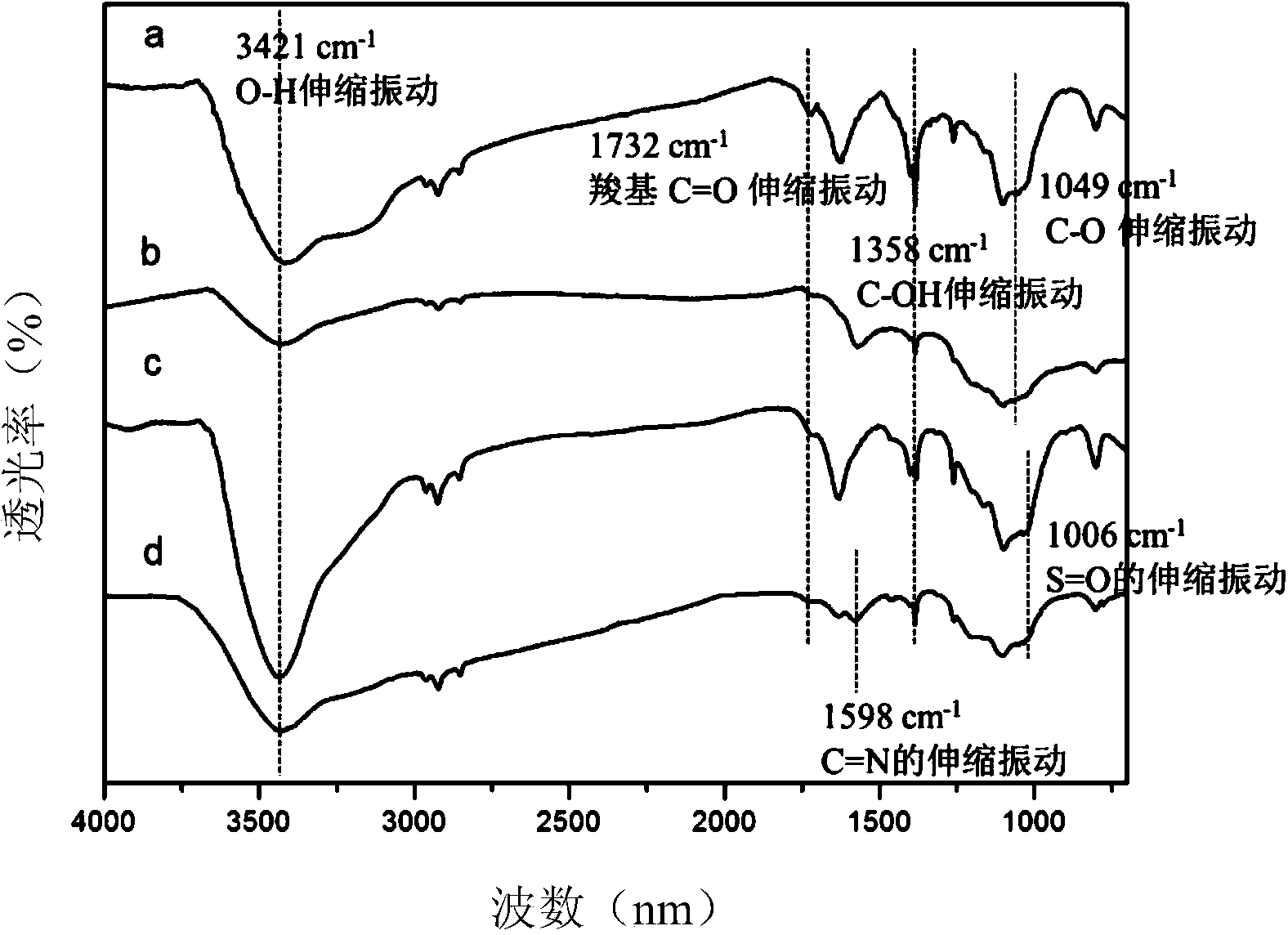
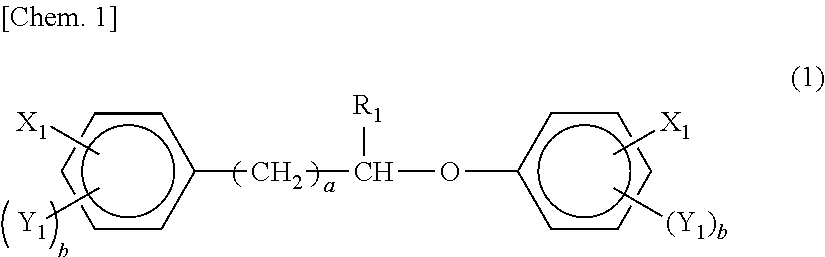
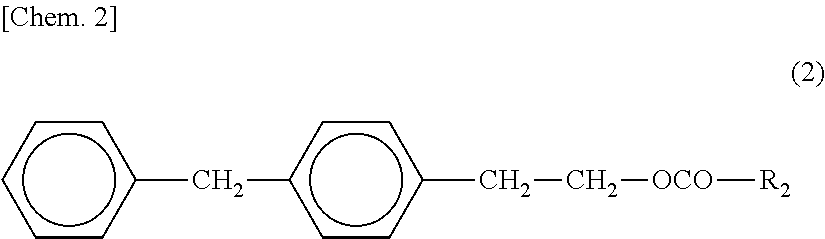
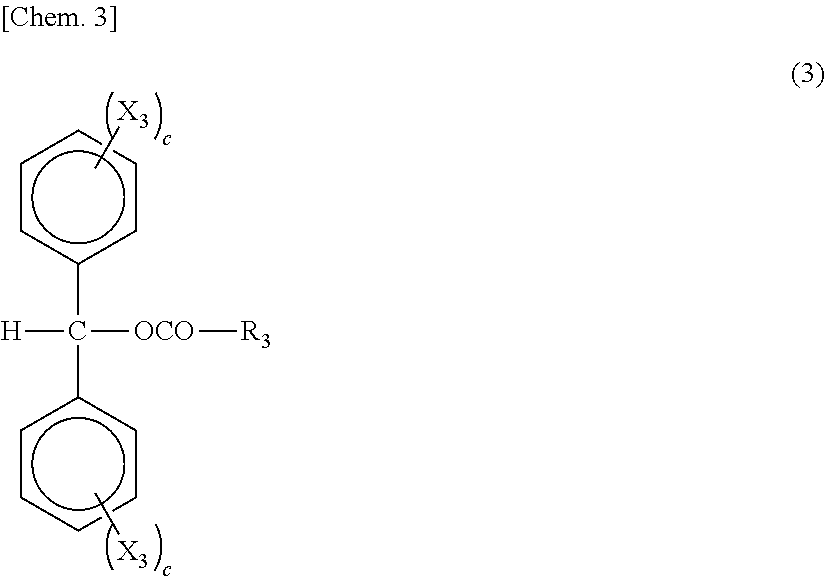Patents
Literature
54 results about "Electron transfer reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In an electron transfer reaction, an element undergoing oxidation loses electrons, whereas an element gaining electrons undergoes reduction. In the aluminum‐oxygen example, the aluminum was oxidized, and the oxygen was reduced because every electron transfer reaction involves simultaneous oxidation and reduction.
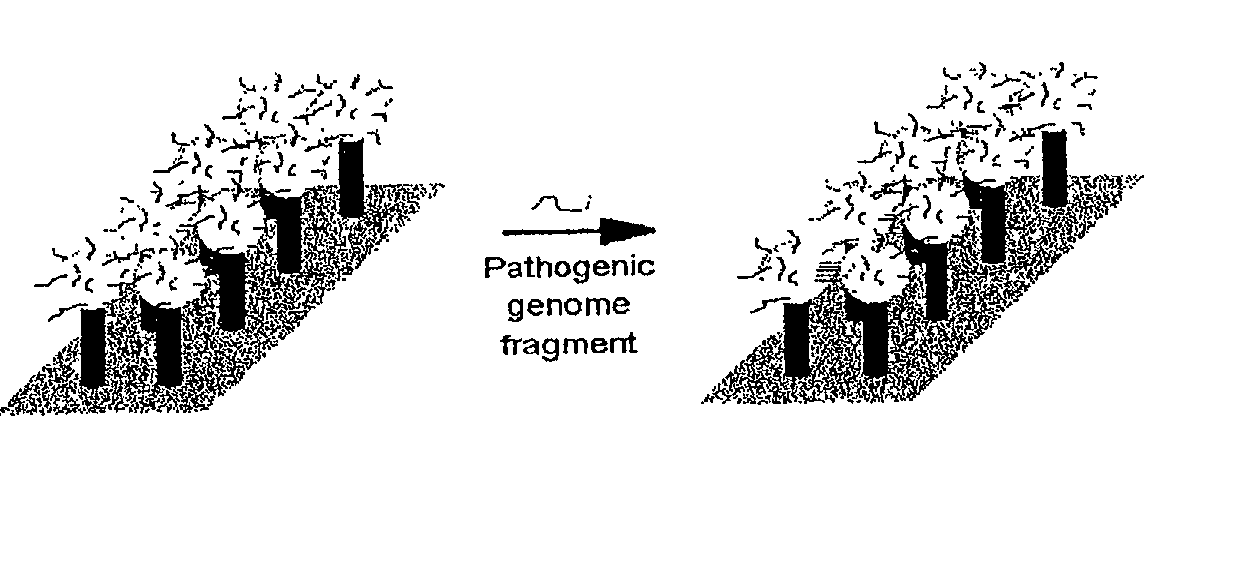
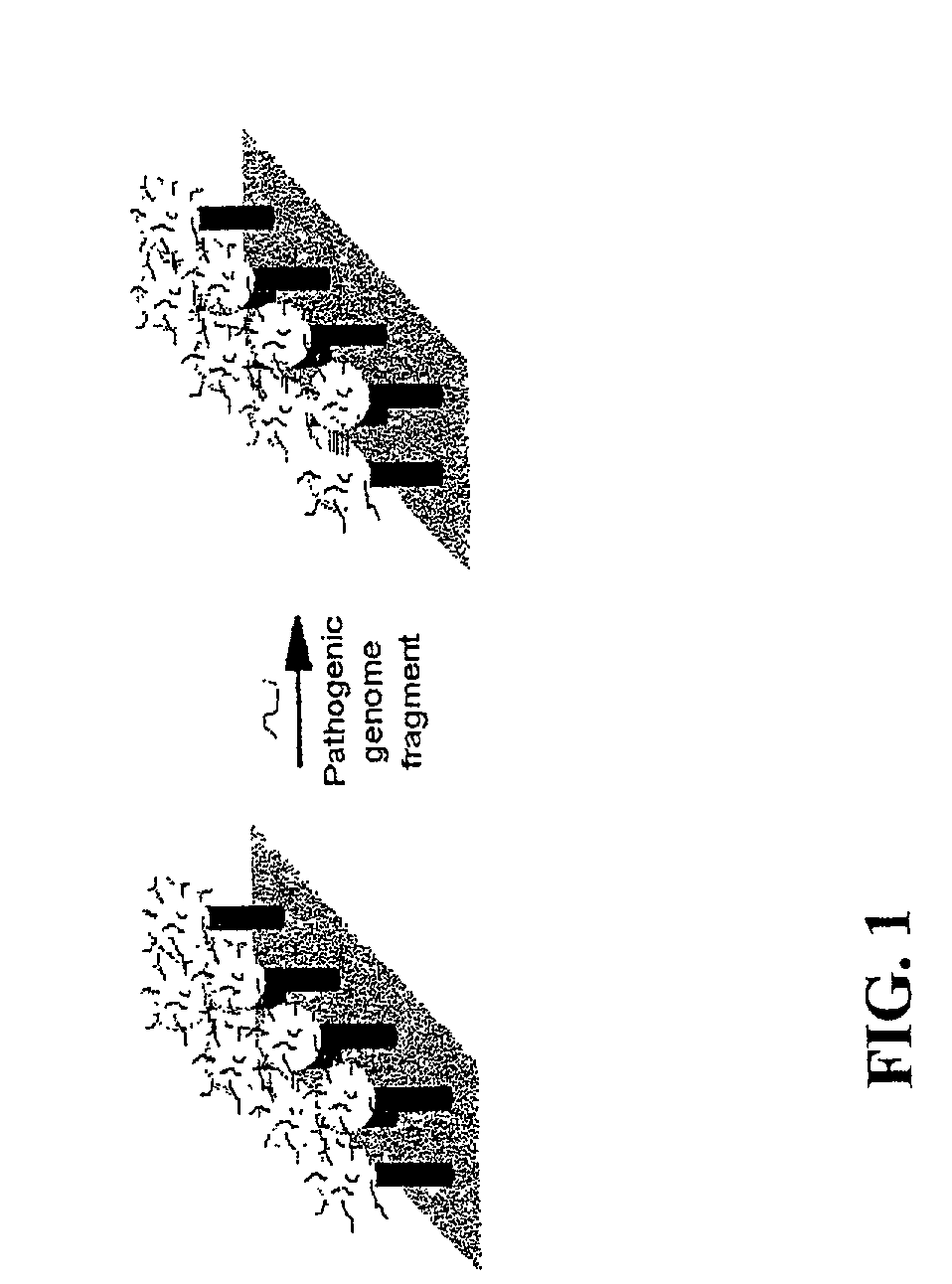
DNA-bridged carbon nanotube arrays
InactiveUS20020172963A1High precisionHigh sensitivityBioreactor/fermenter combinationsMaterial nanotechnologyChemical ligationElectron transfer reactions
A class of biological sensing devices that include a substrate comprising an array of carbon nanotubes (CNTs) to which are chemically attached biological molecules is disclosed. The attached biological molecules are capable of electrical conductivity that is responsive to chemical changes occurring as a result of their interaction with target species. A means for means for using DNA as a material of potential in molecular electronic sensor devices, being primarily based on molecular electron-transfer reaction processes between DNA-binding donors and acceptors is also disclosed, including composition, method of manufacture and their use are described.
Owner:TRUSTEES OF BOSTON COLLEGE THE
DNA-bridged carbon nanotube arrays
InactiveUS6958216B2High sensitivityImprove portabilityImmobilised enzymesBioreactor/fermenter combinationsChemical ligationElectron transfer reactions
A class of biological sensing devices that include a substrate comprising an array of carbon nanotubes (CNTs) to which are chemically attached biological molecules is disclosed. The attached biological molecules are capable of electrical conductivity that is responsive to chemical changes occurring as a result of their interaction with target species. A means for means for using DNA as a material of potential in molecular electronic sensor devices, being primarily based on molecular electron-transfer reaction processes between DNA-binding donors and acceptors is also disclosed, including composition, method of manufacture and their use are described.
Owner:TRUSTEES OF BOSTON COLLEGE THE
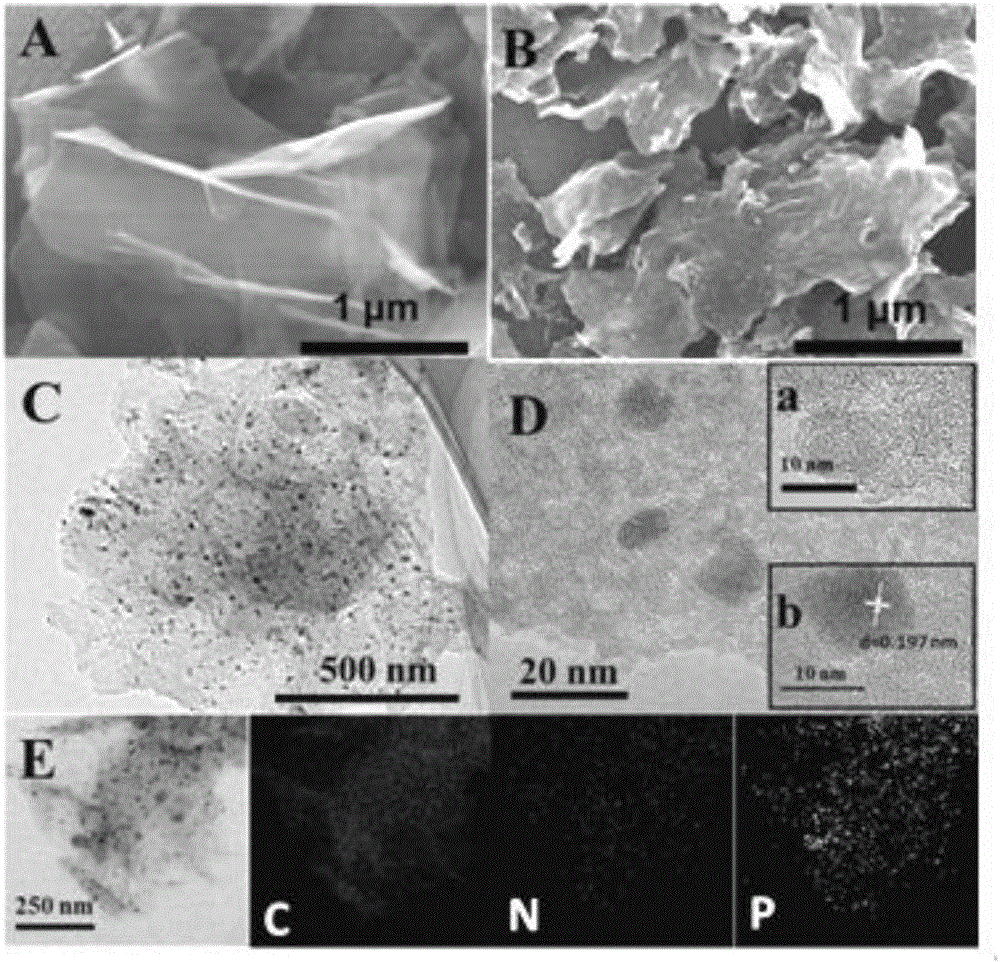
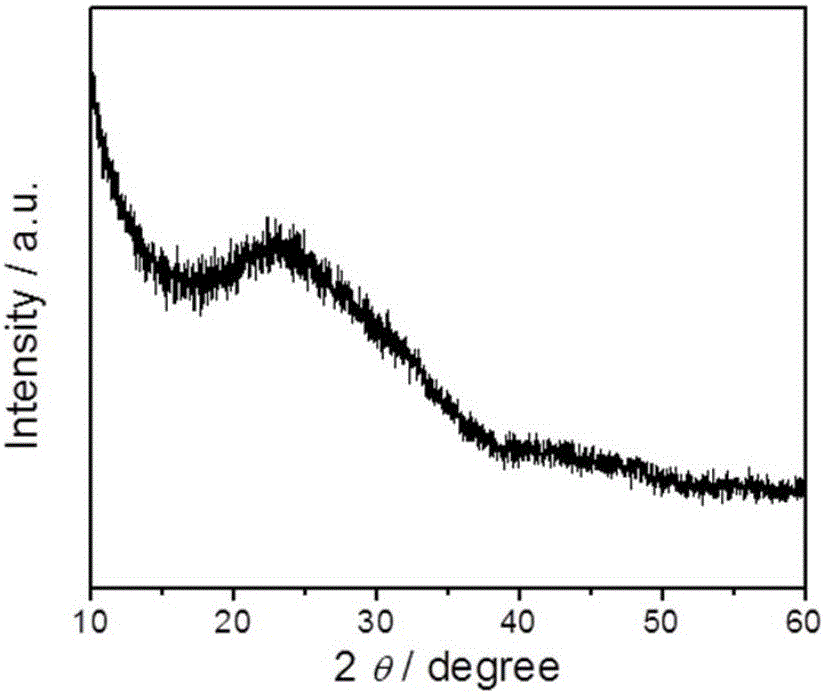
Preparation method of nitrogen-phosphorus co-doped carbon nanosheet and application of preparation method
ActiveCN105762376APositive onset potentialIncrease current densityMaterial nanotechnologyCell electrodesHigh current densityElectron transfer reactions
The invention discloses a preparation method of a nitrogen-phosphorus co-doped carbon nanosheet and application of the preparation method.Nitrogen-phosphorus co-doped carbon nanosheet catalyst material is prepared by: calcining at high temperature, template-free one-step self-assembled precursors: melamine and phytic acid.Oxygen reduction tests show that prepared N-P / CNS-1000 shows corrected initial potential and higher current density, represents four-electron transfer reaction, can match with commercial Pt / C and shows both excellent ethanol resistance and long-range stability in alkali solutions.
Owner:QINGDAO UNIV
Photoelectric Converter, and Transparent Conductive Substrate for the same
InactiveUS20080083452A1Improve photoelectric conversion efficiencyLowering of conversionLight-sensitive devicesPhotovoltaic energy generationSemiconductor electrodeElectron transfer reactions
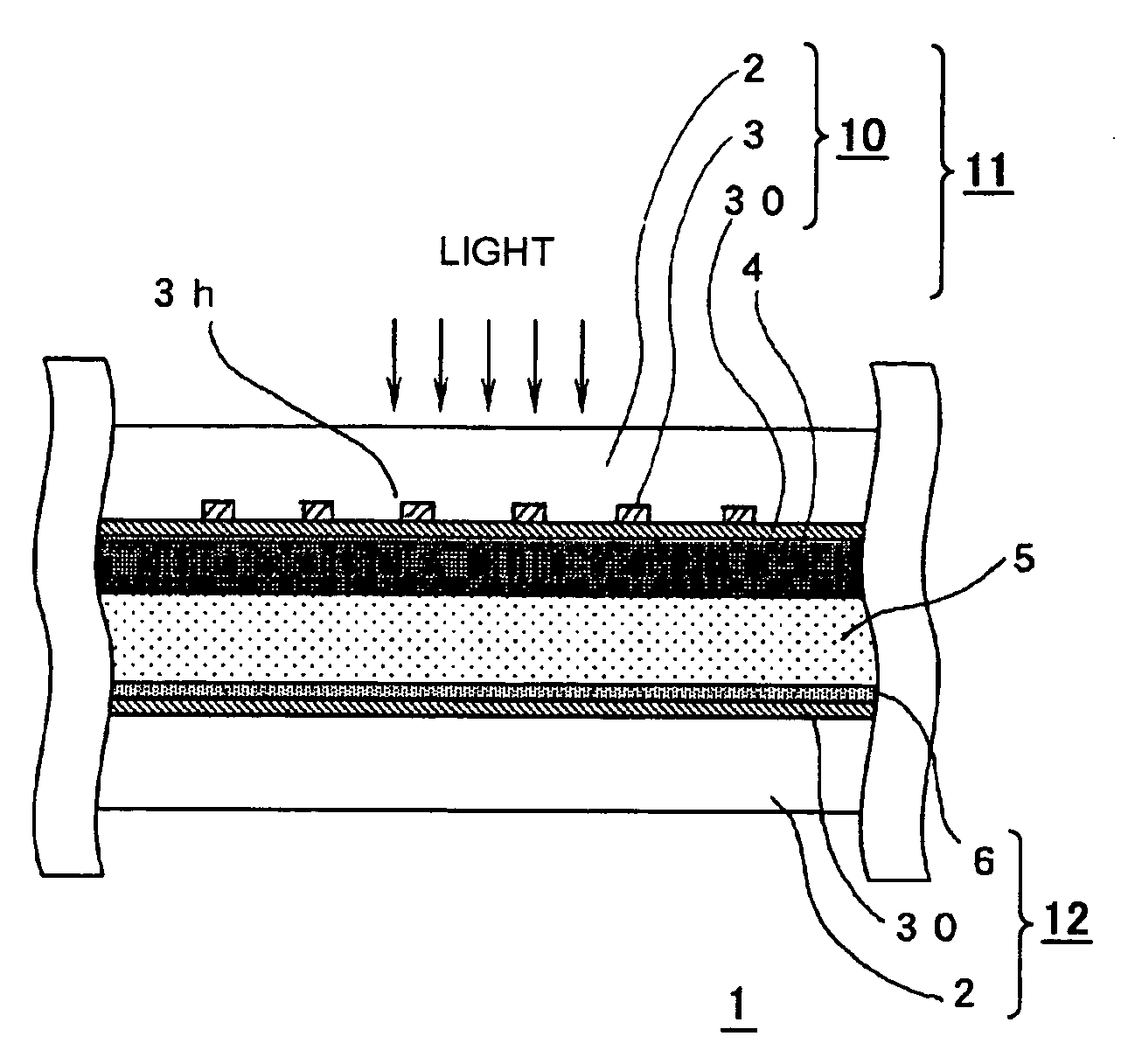
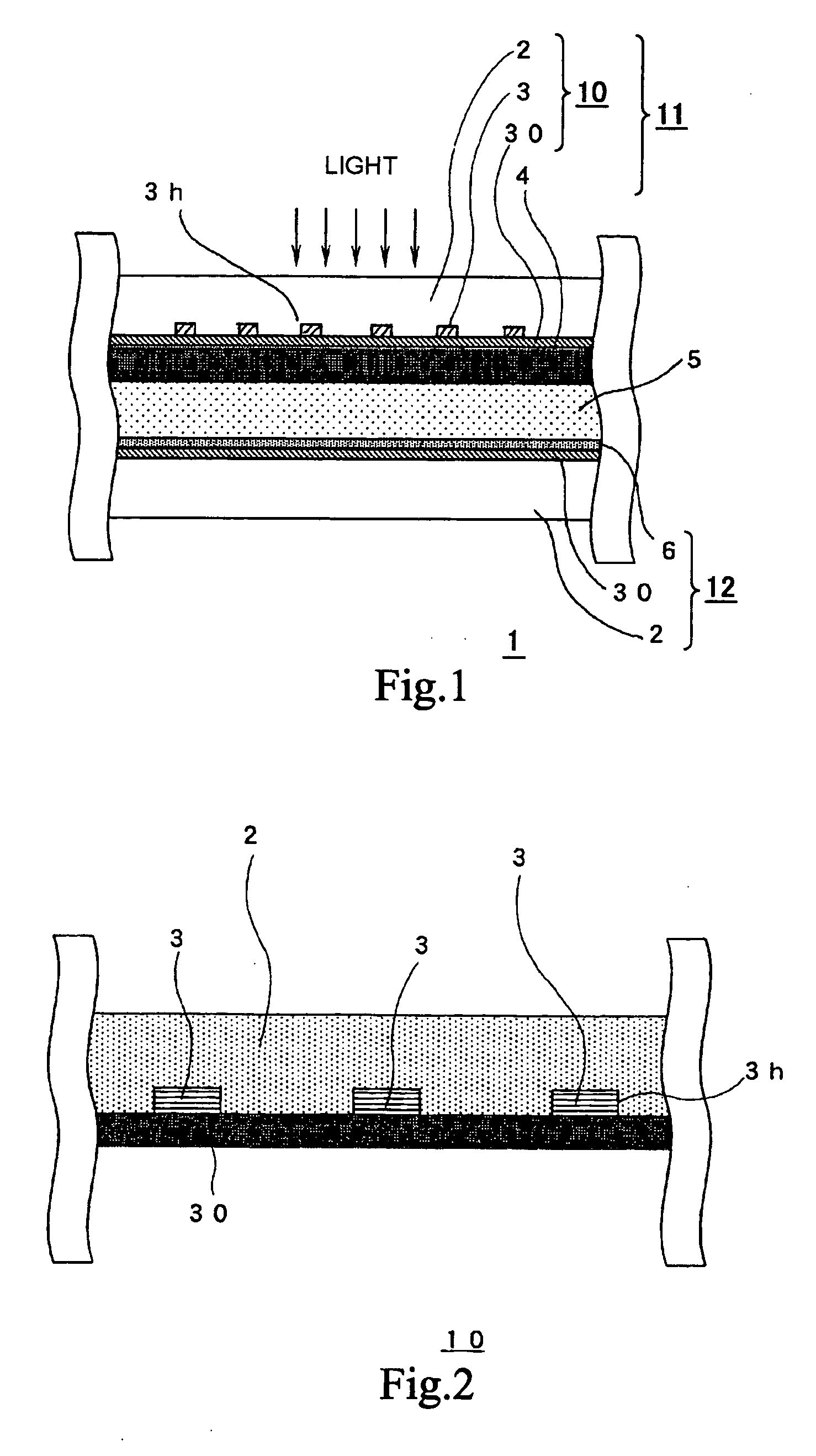
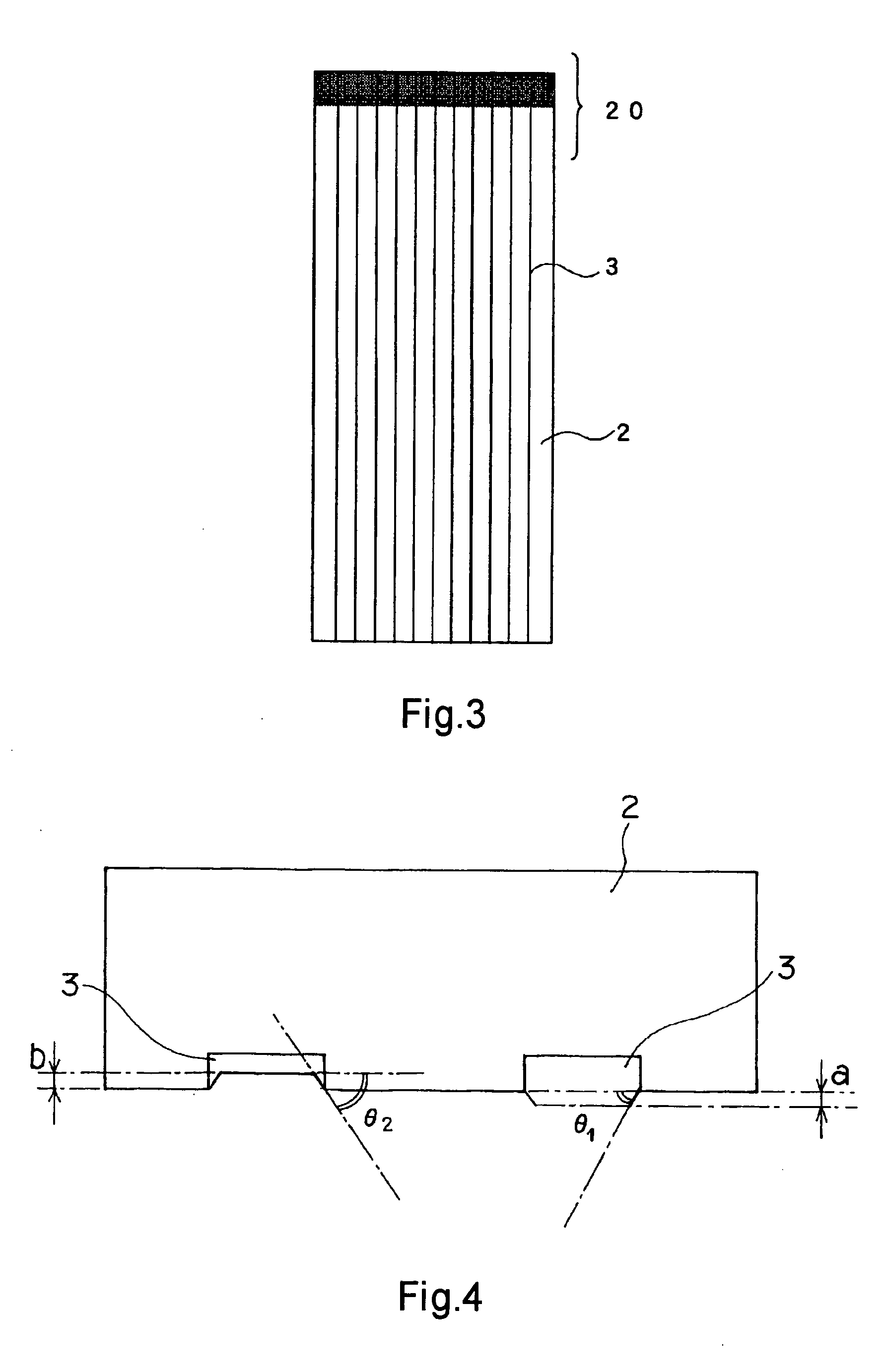
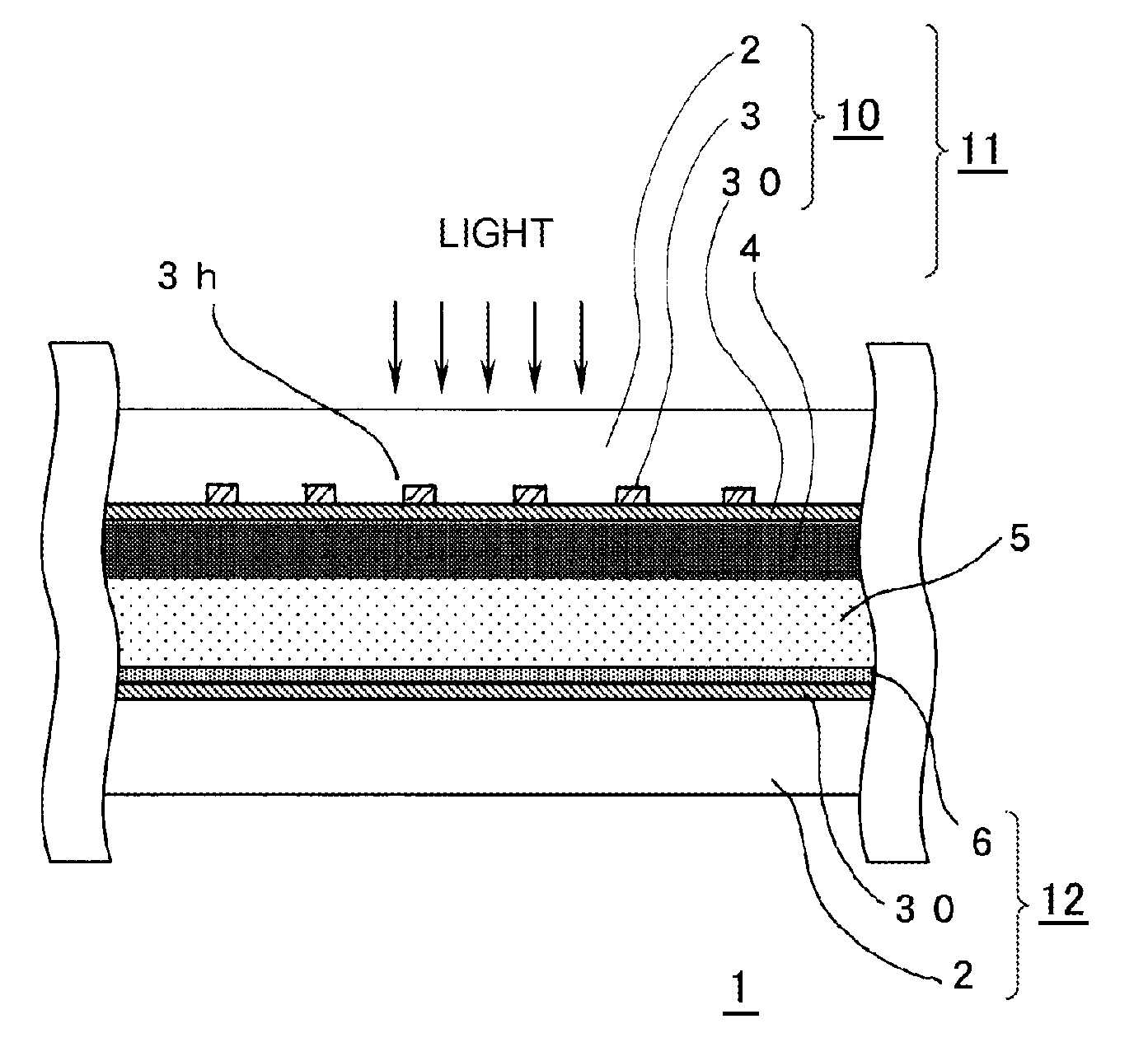
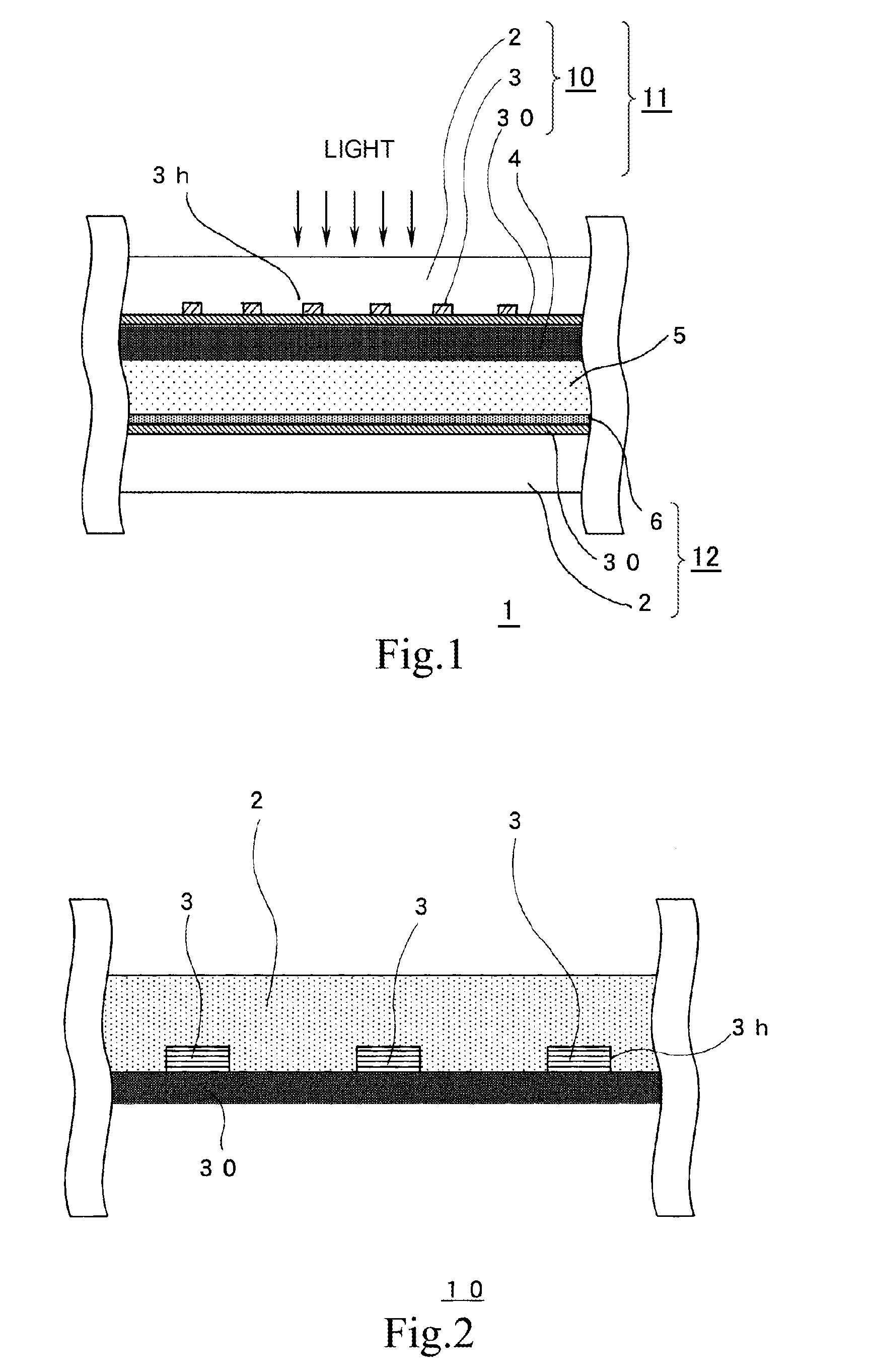
A highly durable photoelectric converter with excellent photoelectric conversion efficiency is prevented from resistance loss or lowering of photoelectric conversion efficiency and free from problems of corrosion and reverse electron transfer reaction. Specifically disclosed is a photoelectric converter (1) comprising a semiconductor electrode (11), a counter electrode (12), and an electrolyte layer (5) arranged between the electrodes. The semiconductor electrode (11) includes a transparent conductive substrate (10) including a transparent base (2), a conductive interconnection layer (3), and a metal oxide layer (30), and a semiconductor particle layer (4) arranged on the transparent conductive substrate (10). The transparent base (2) of the transparent conductive substrate (10) has a trench (3h) on one surface, and the conductive interconnection layer (3) is embedded in this trench (3h).
Owner:SONY CORP
Ion fragmentation by electron transfer in ion traps
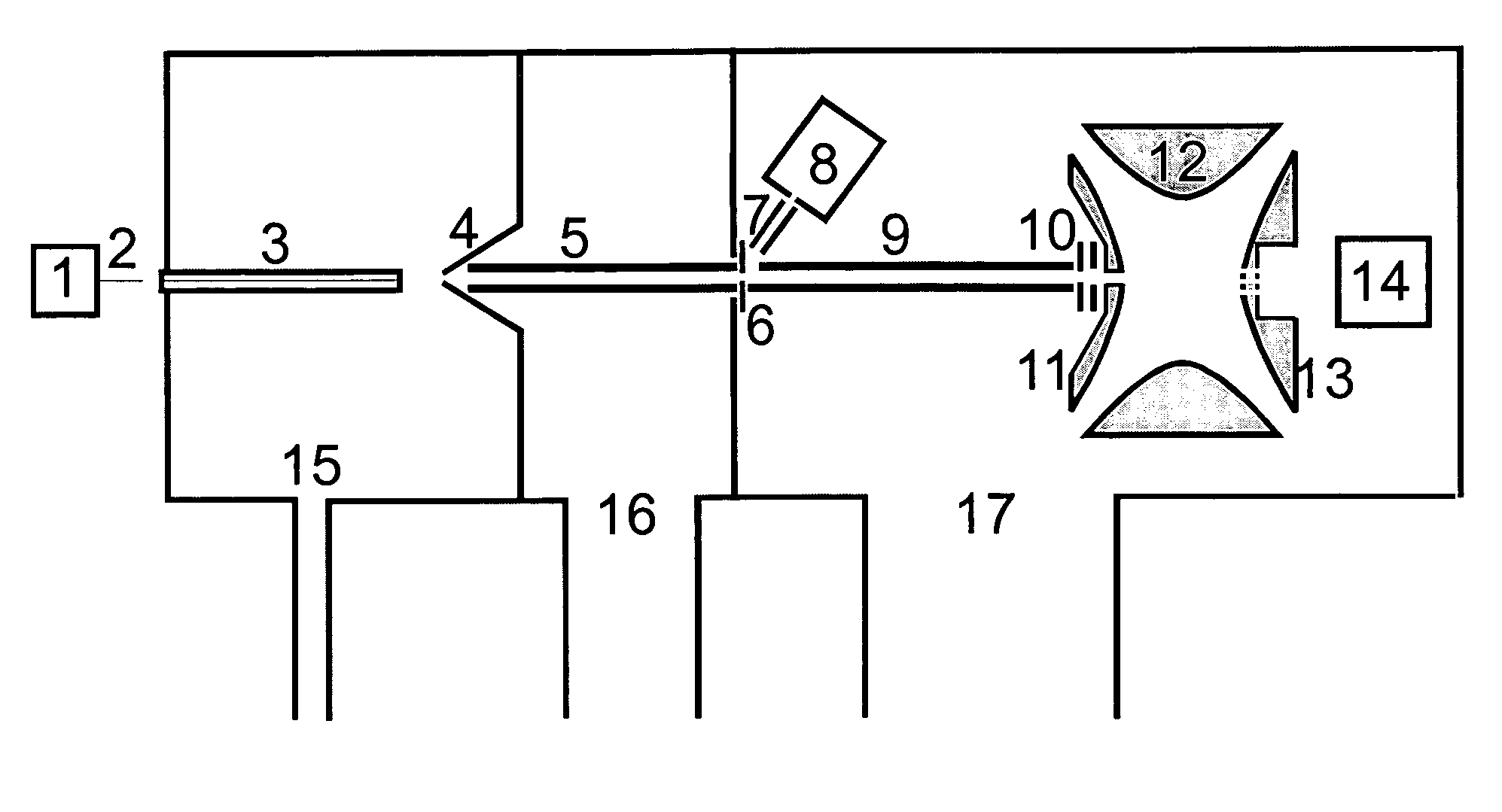
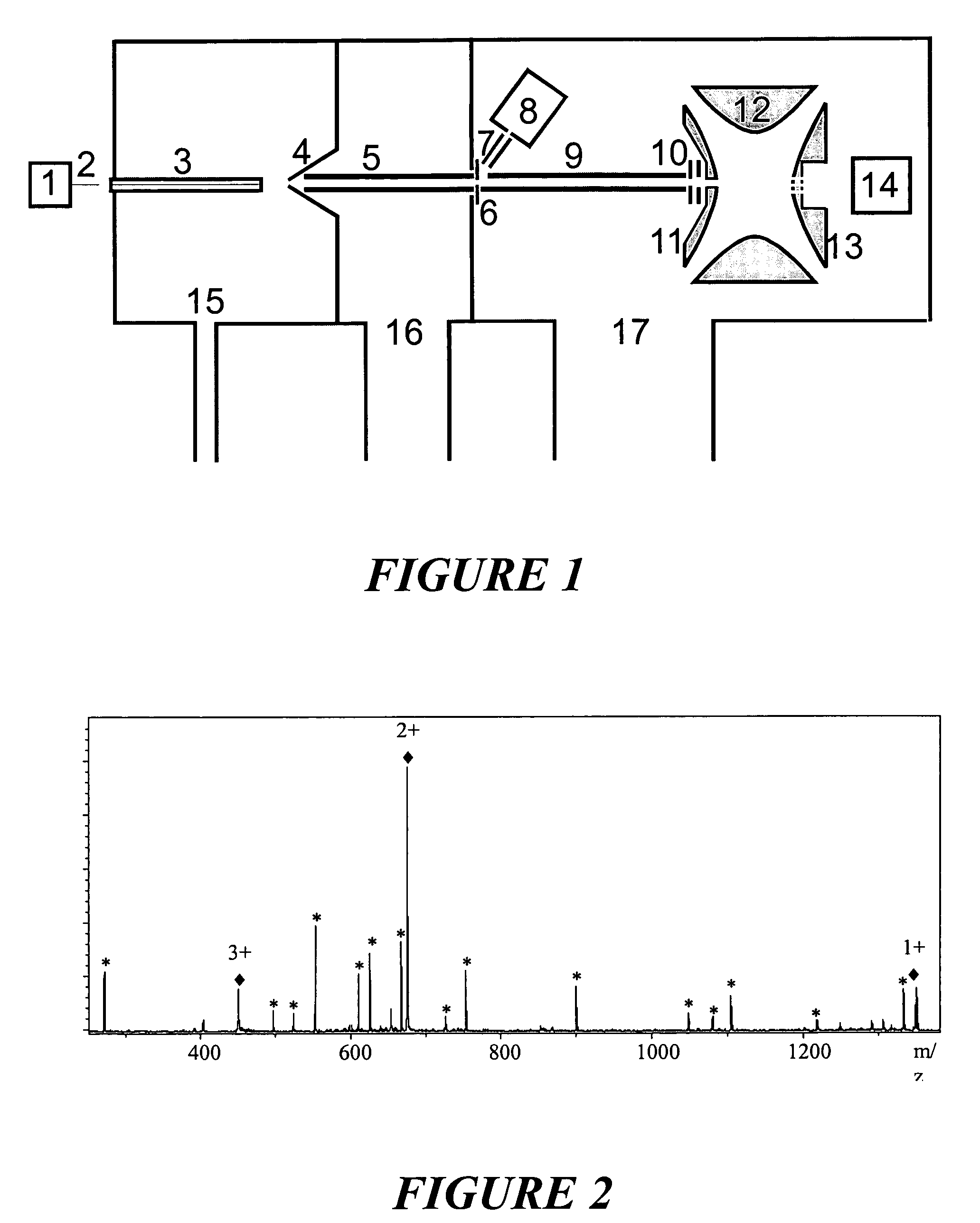
The invention relates to a method and instrument for the fragmentation of large molecular analyte ions, preferably biopolymer ions, by reactions between multiply charged positive analyte ions and negative reactant ions in RF quadrupole ion traps. Some of these reactions involve electron transfer reactions with subsequent dissociation of the biopolymer analyte ions, and some involve the loss of a proton, leading to stable product ions. The invention can use any type of ion traps, particularly three-dimensional RF quadrupole ion traps, for the reactions between positive and negative ions. The fragmentation yield can be increased because ions that remain stable as radical cations after transfer of an electron are further fragmented by collisionally induced fragmentation, forming fragment ions that are typical of electron transfer, and not those typical of collisionally induced fragmentation. The invention preferentially introduces positive ions and negative ions into the ion trap sequentially through the same aperture.
Owner:BRUKER DALTONIK GMBH & CO KG
Ion fragmentation by electron transfer in ion traps
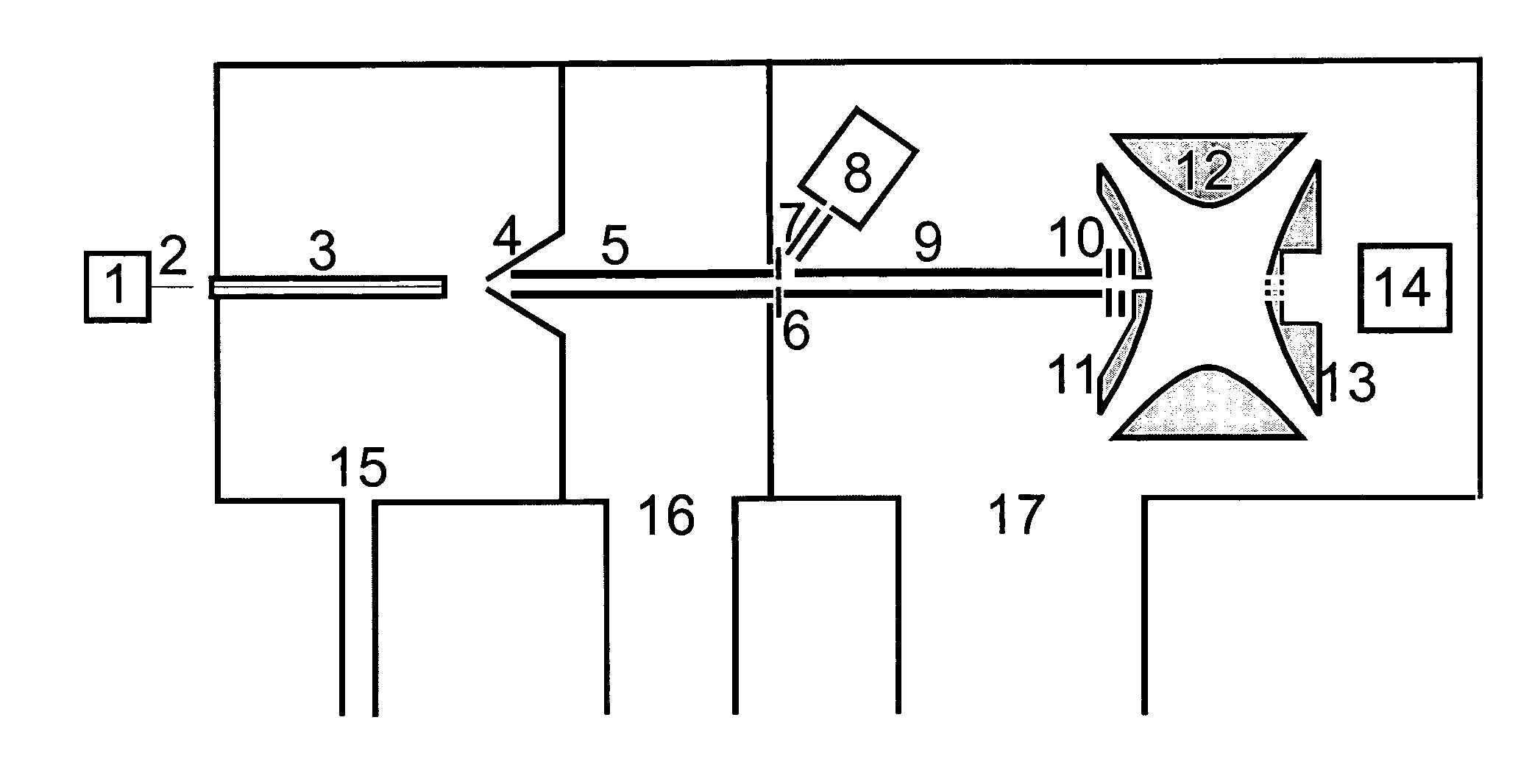
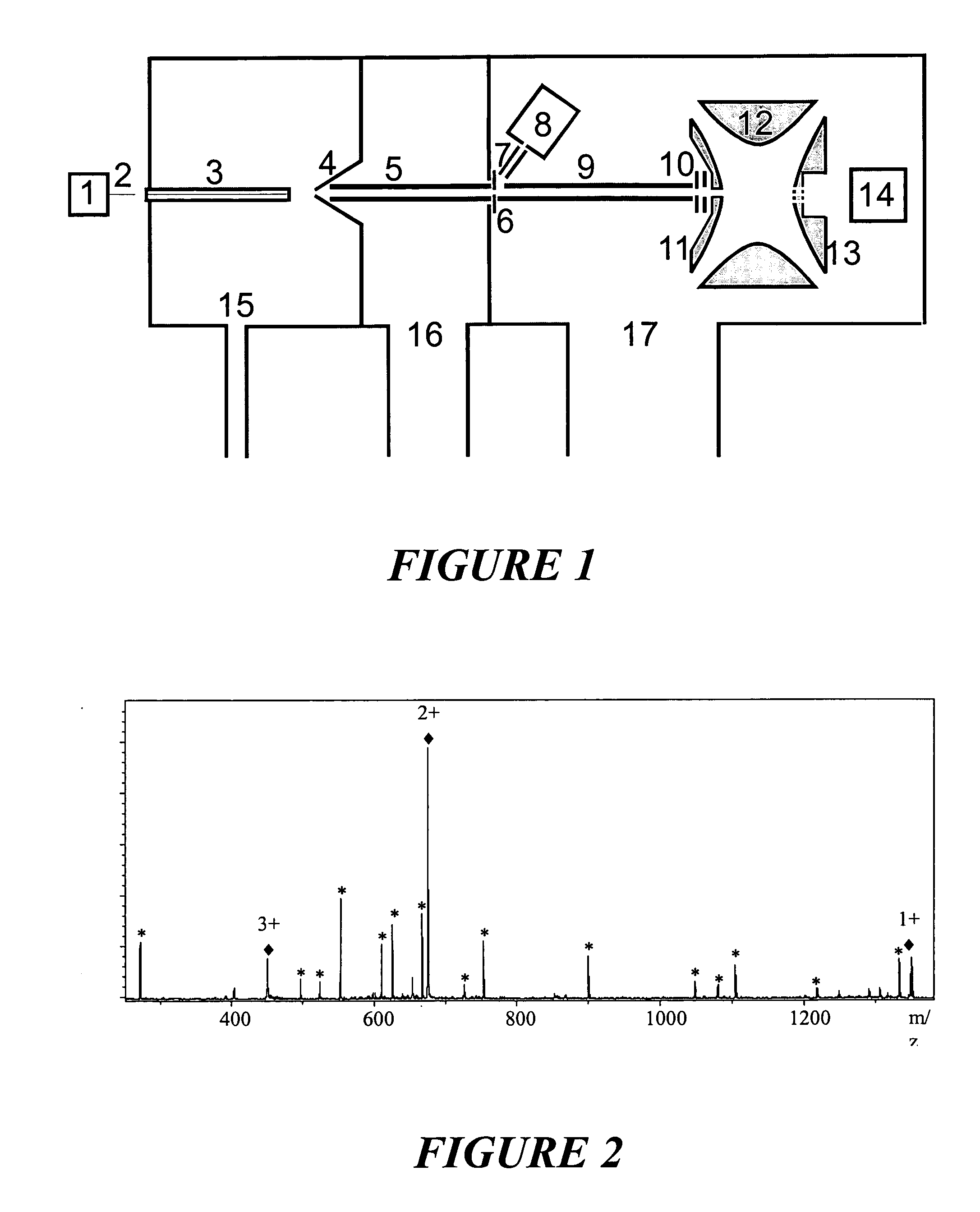
ActiveUS20060186331A1Isotope separationParticle separator tube detailsIon trap mass spectrometryAnalyte
The invention relates to a method and instrument for the fragmentation of large molecular analyte ions, preferably biopolymer ions, by reactions between multiply charged positive analyte ions and negative reactant ions in RF quadrupole ion traps. Some of these reactions involve electron transfer reactions with subsequent dissociation of the biopolymer analyte ions, and some involve the loss of a proton, leading to stable product ions. The invention can use any type of ion traps, particularly three-dimensional RF quadrupole ion traps, for the reactions between positive and negative ions. The fragmentation yield can be increased because ions that remain stable as radical cations after transfer of an electron are further fragmented by collisionally induced fragmentation, forming fragment ions that are typical of electron transfer, and not those typical of collisionally induced fragmentation. The invention preferentially introduces positive ions and negative ions into the ion trap sequentially through the same aperture.
Owner:BRUKER DALTONIK GMBH & CO KG
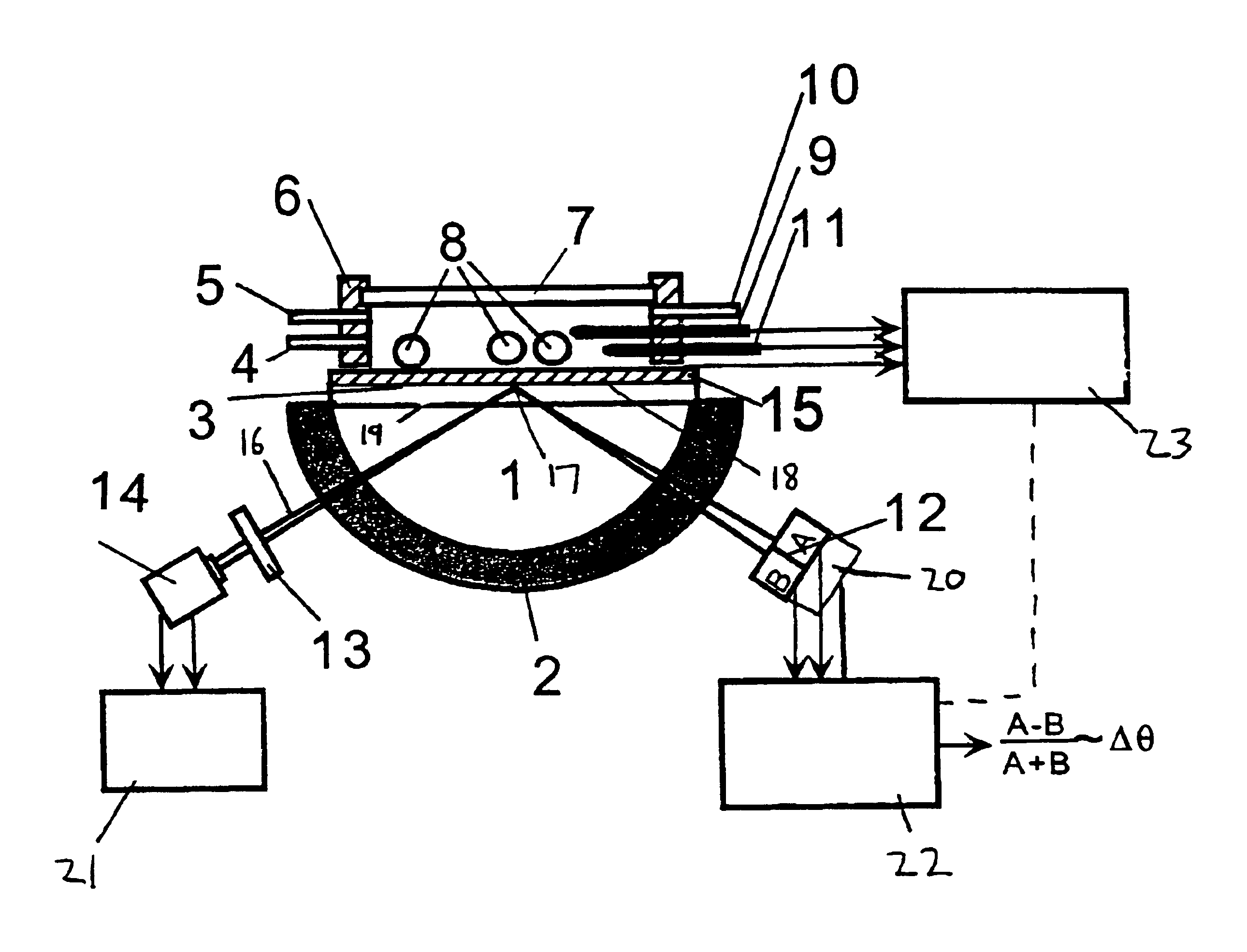
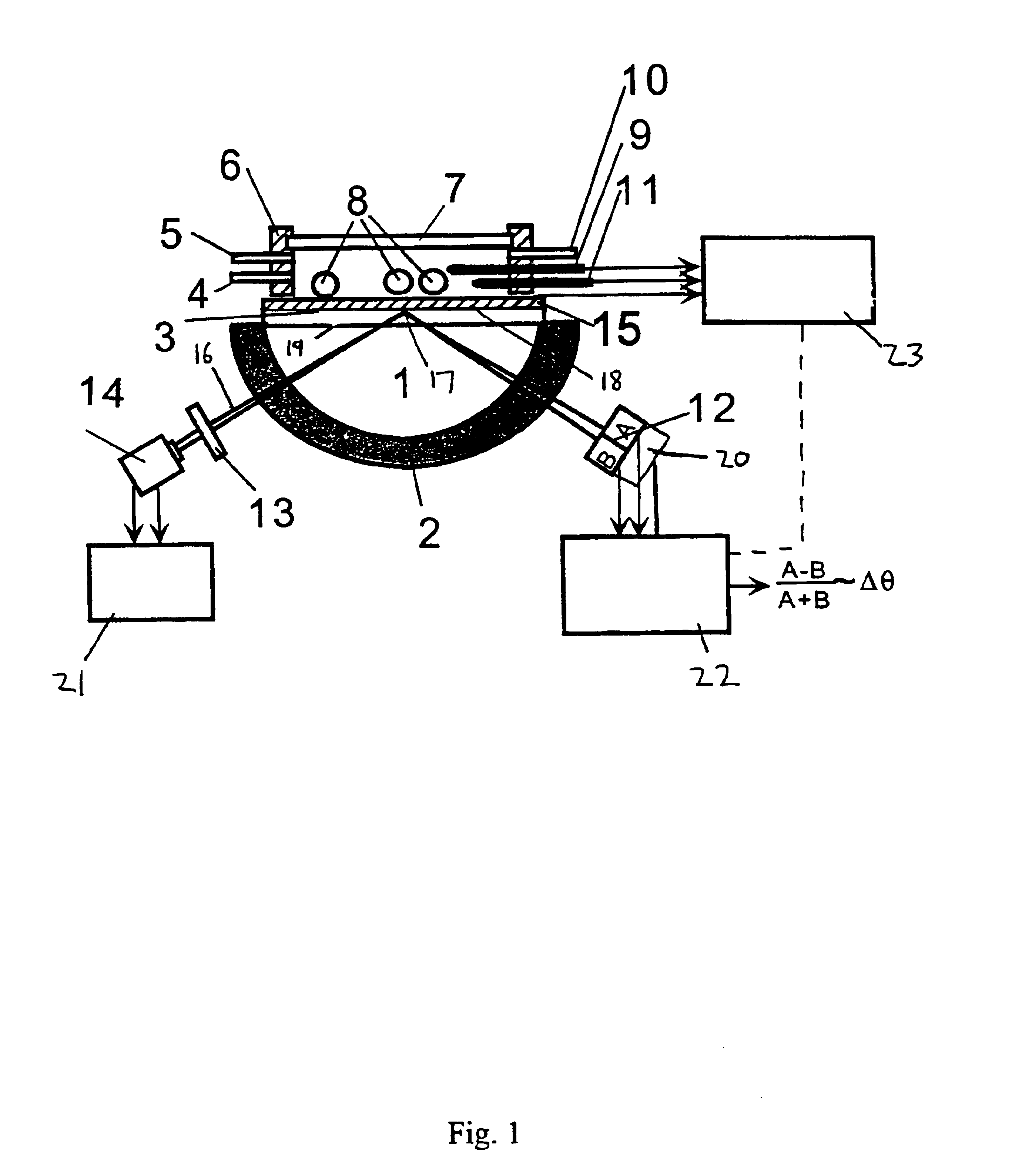
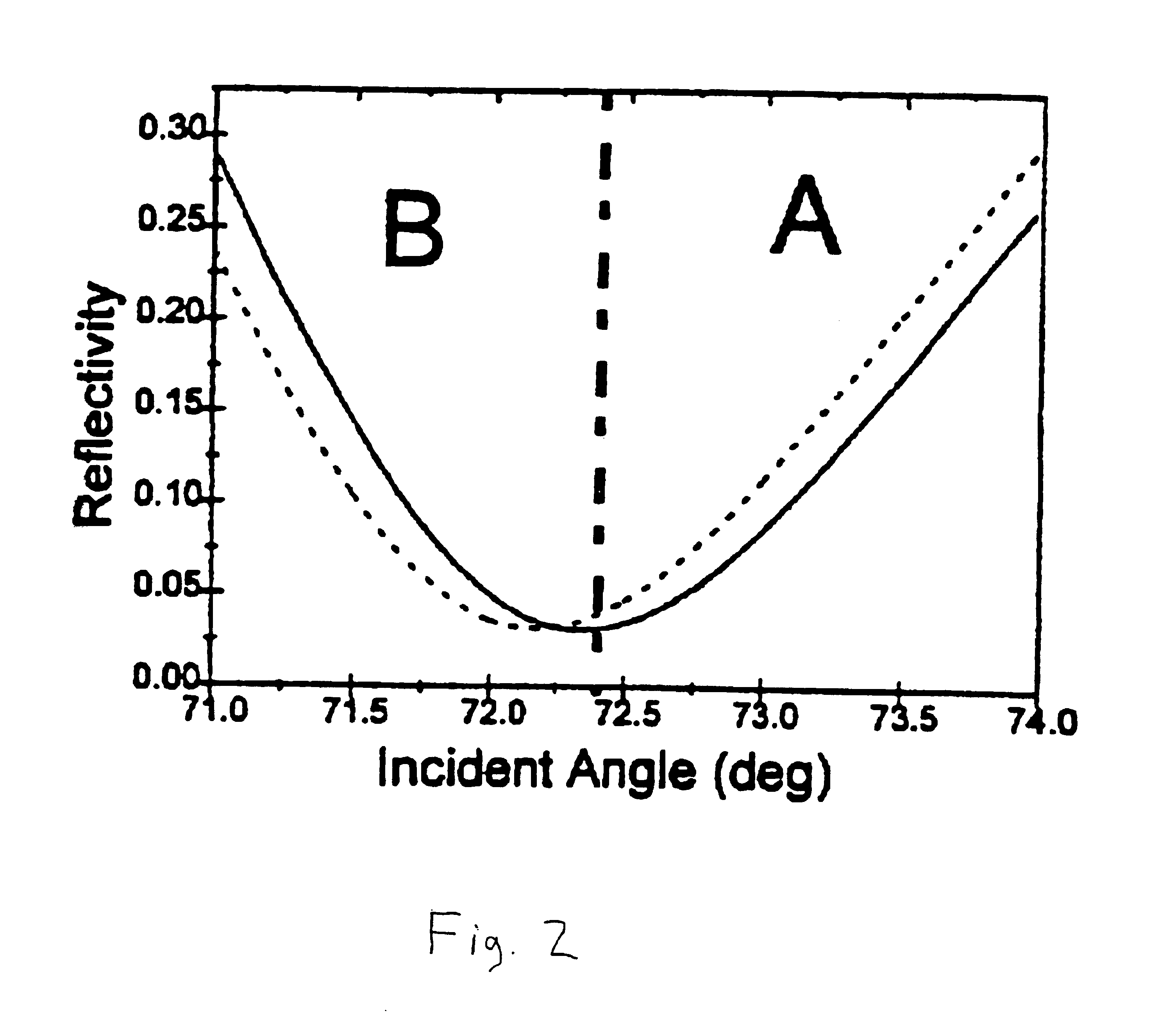
Surface plasmon resonance detection with high angular resolution and fast response time
InactiveUS6784999B1Scattering properties measurementsElectron transfer reactionsDifferential signaling
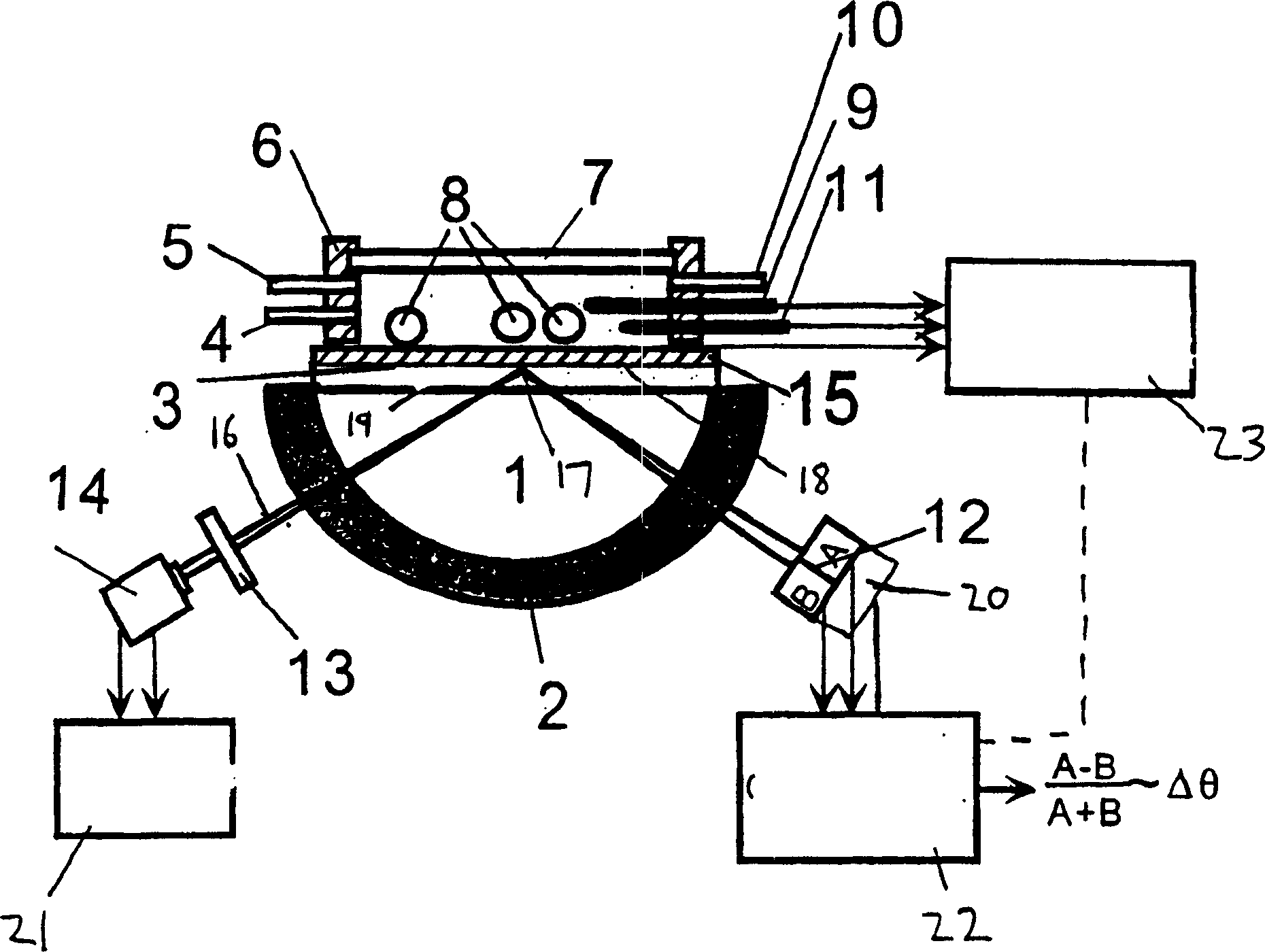
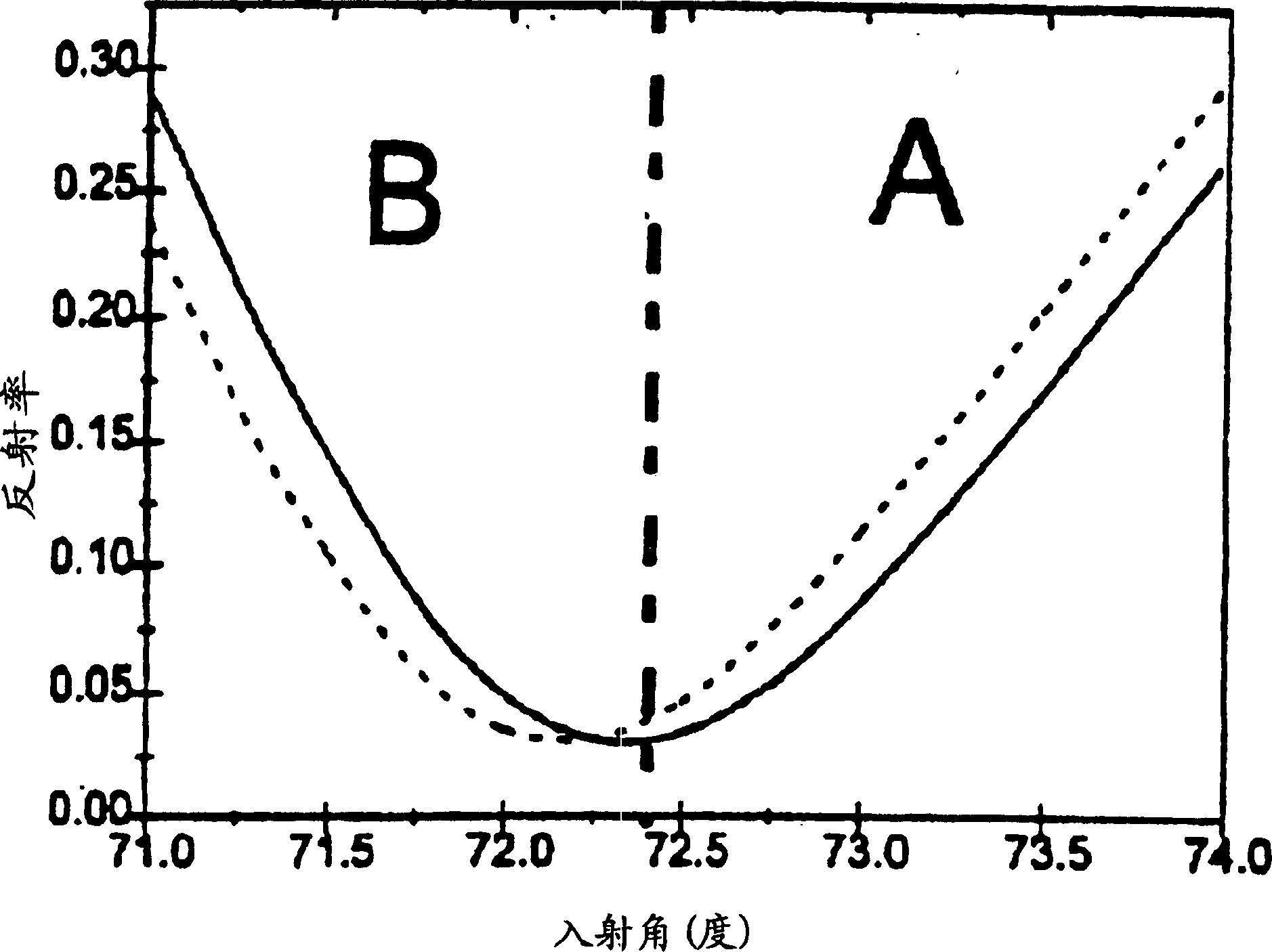
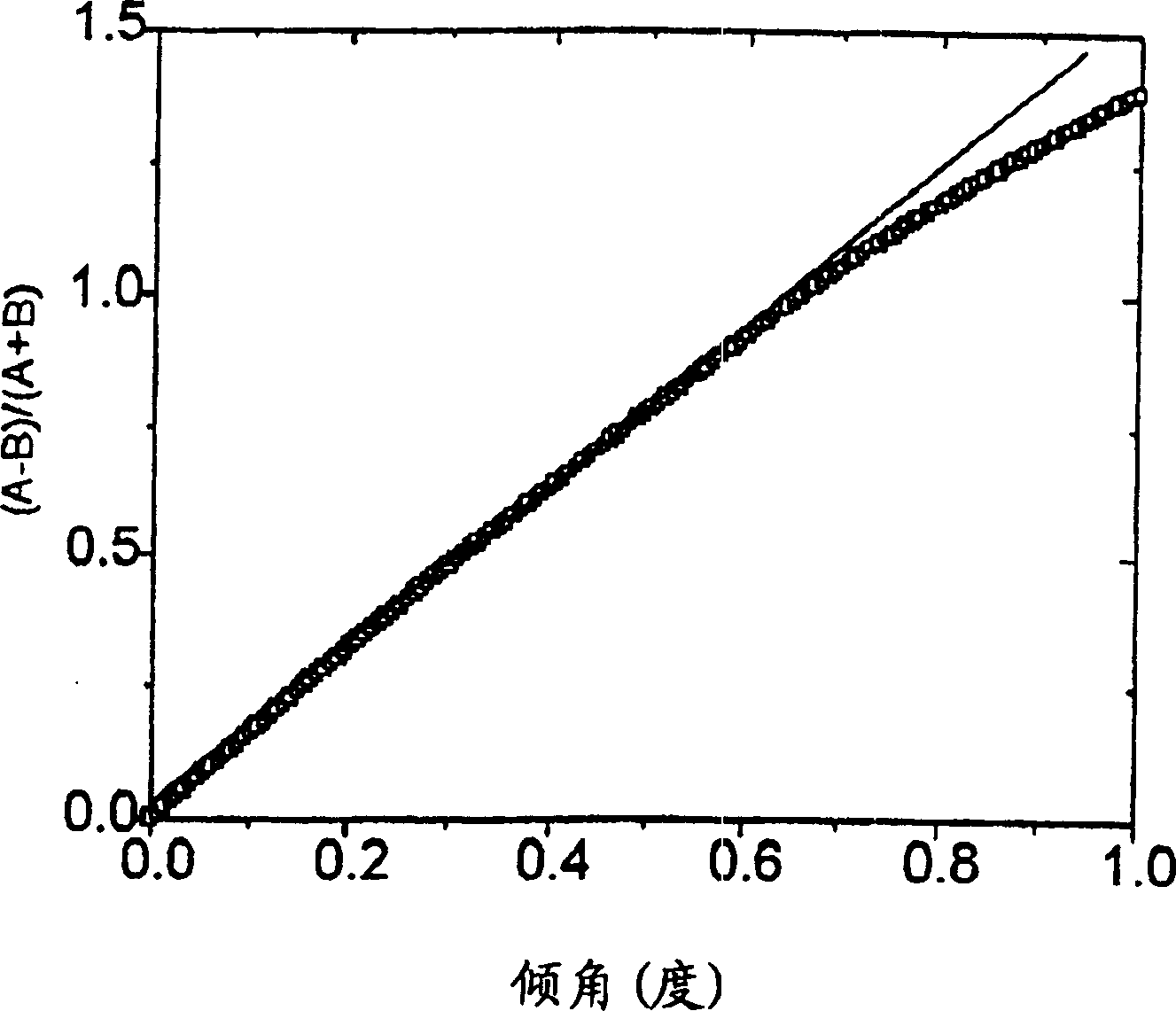
A device and method of detecting surface plasmon resonance for sensing molecules or conformational changes in molecules with high resolution and fast response time is disclosed. Light from a light source (14) is focused through a prism onto a metal thin film (15) on which sample molecules to be detected are adsorbed. The total internal reflection of the laser / incident light is collected with a differential position or intensity sensitive photo-detecting device instead of a single cell or an array of photo-detectors (12) that are widely used in previous works. The ratio of the differential signal to the sum signal of the differential position or intensity sensitive photo-detecting device (12) provides an accurate measurement of the shift in the surface plasmon resonance angle caused by the adsorption of molecules onto the metal films (15) or by conformational changes in the adsorbed molecules. The present invention requires no numerical fitting to determine the resonant angle and the setup is compact and immune to background light, The methods and sensors of this invention can be used in numerous biological, biochemical, and chemical applications such as measuring subtle conformational changes in molecules and electron transfer reactions can be studied.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Gas separations with redox-active metal-organic frameworks
ActiveUS20130053585A1Incredible separation propertyNitrous oxide capturePreparation by oxidation reactionsHigh densityElectron transfer reactions
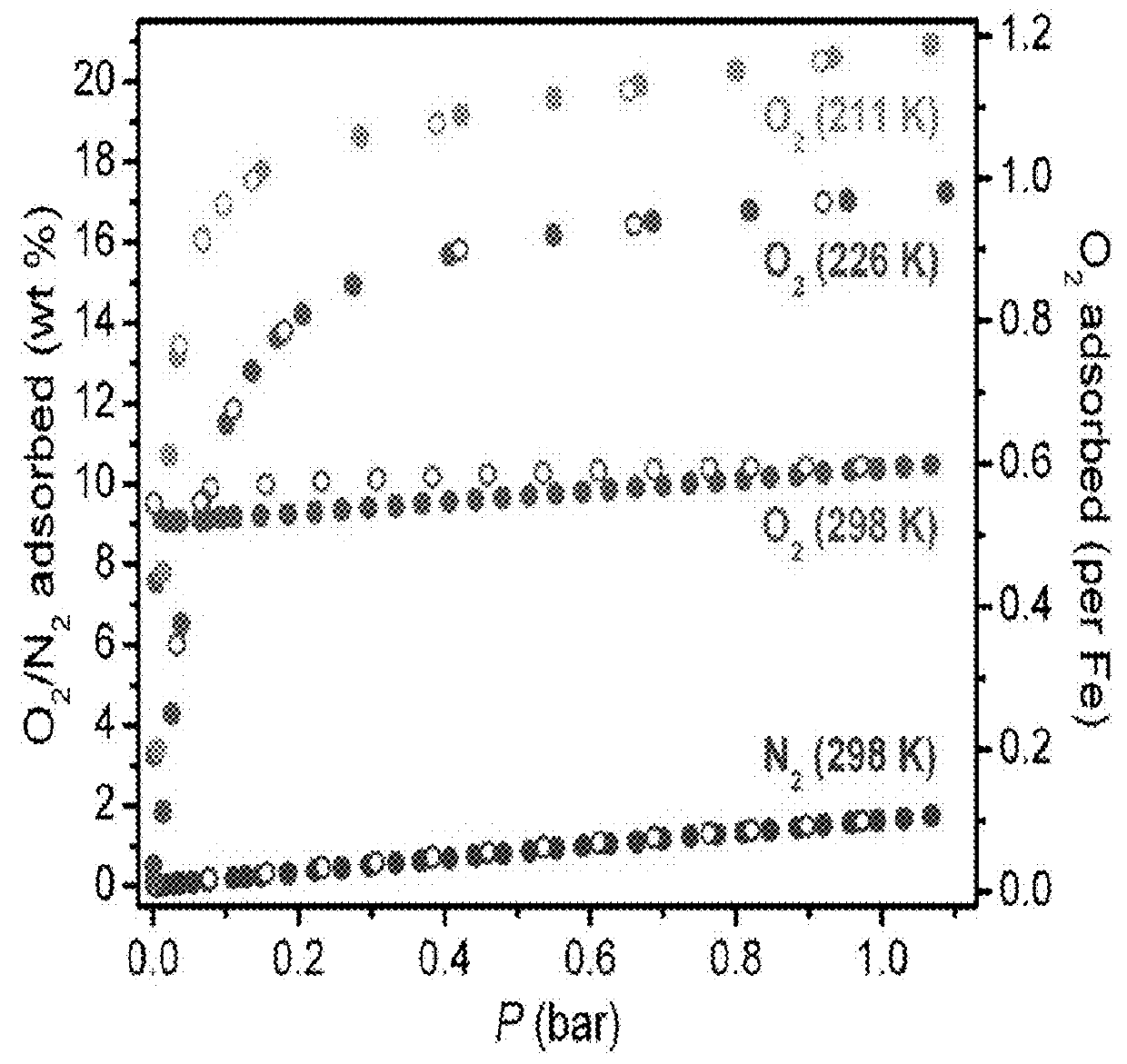
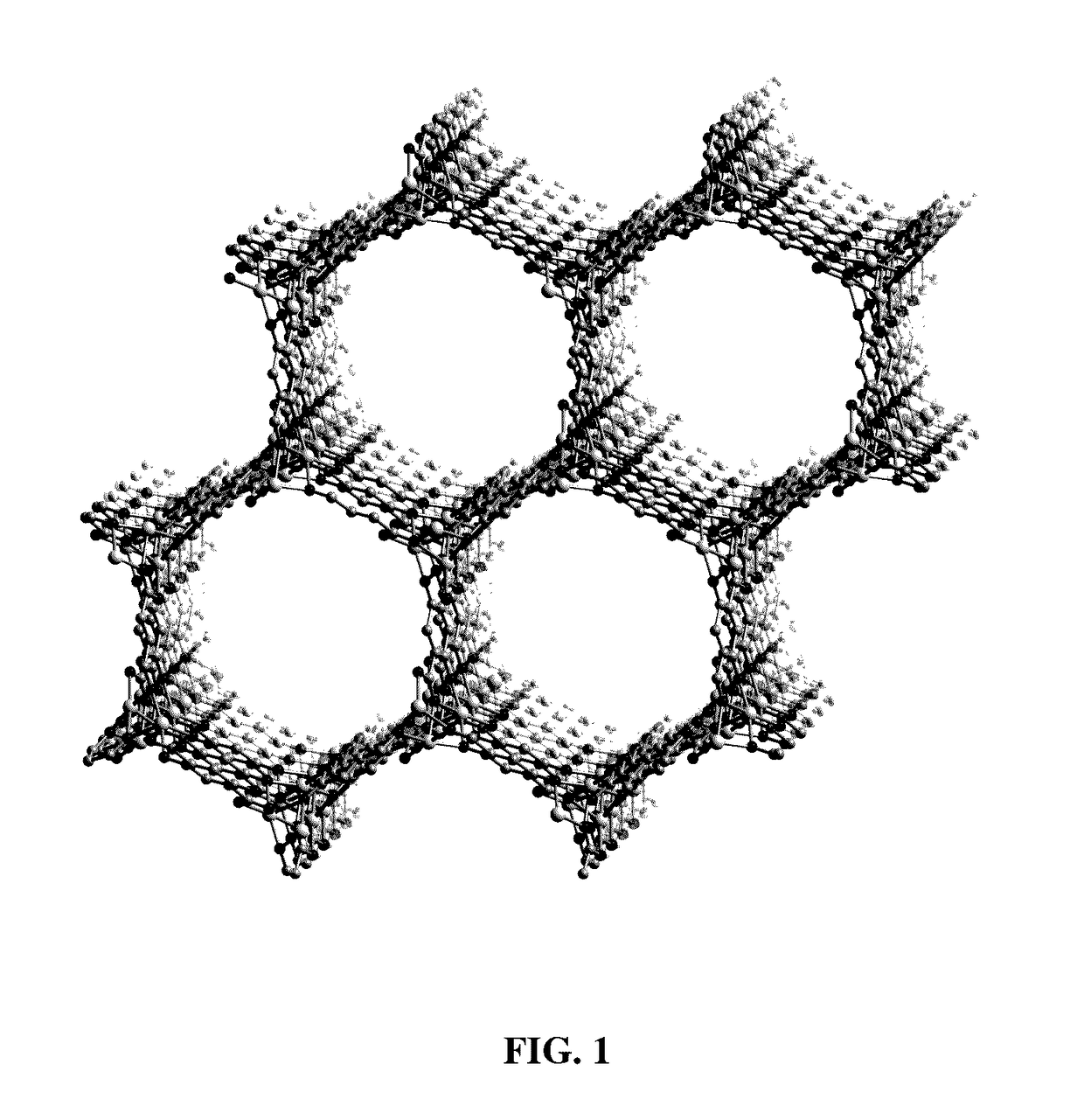
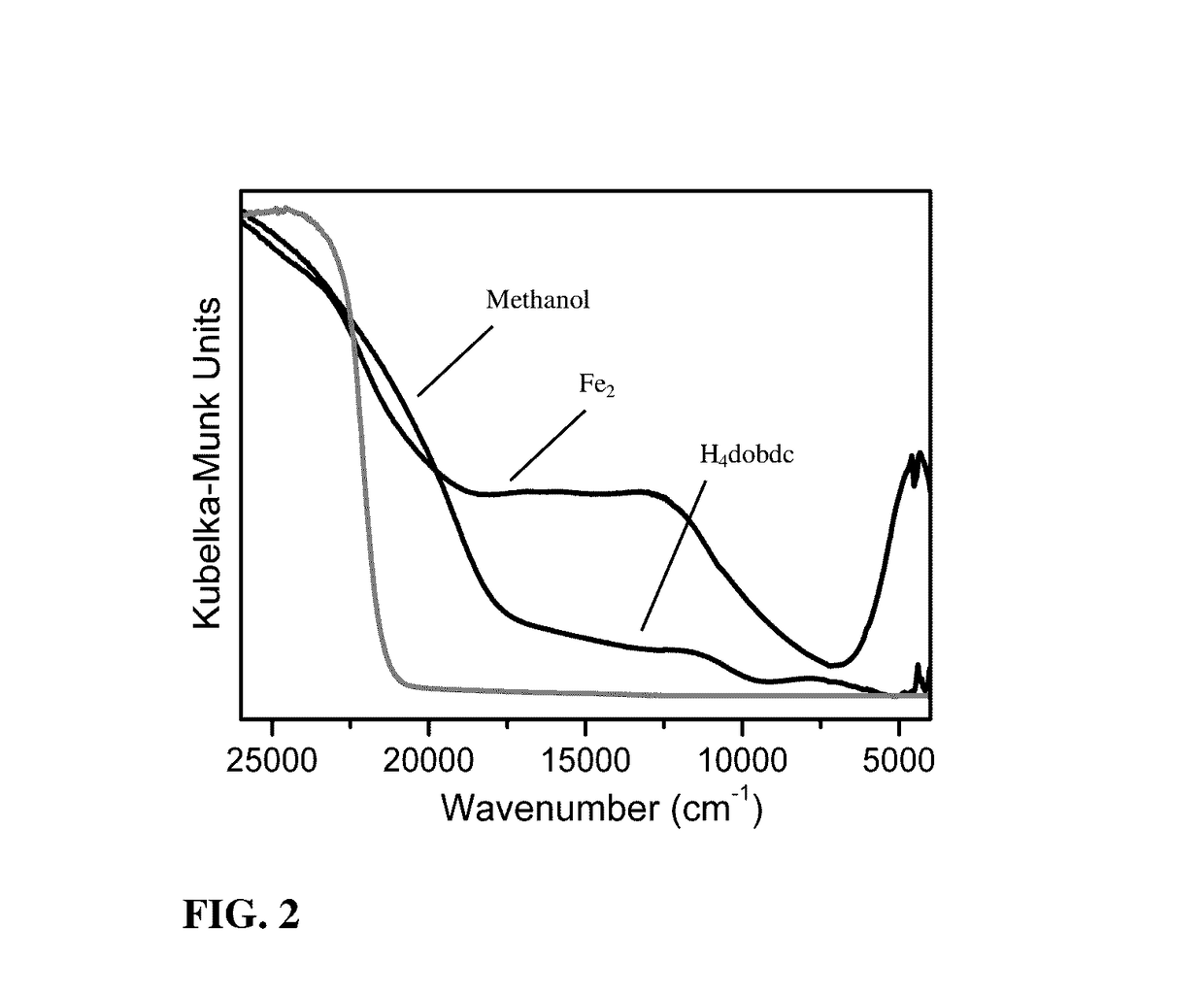
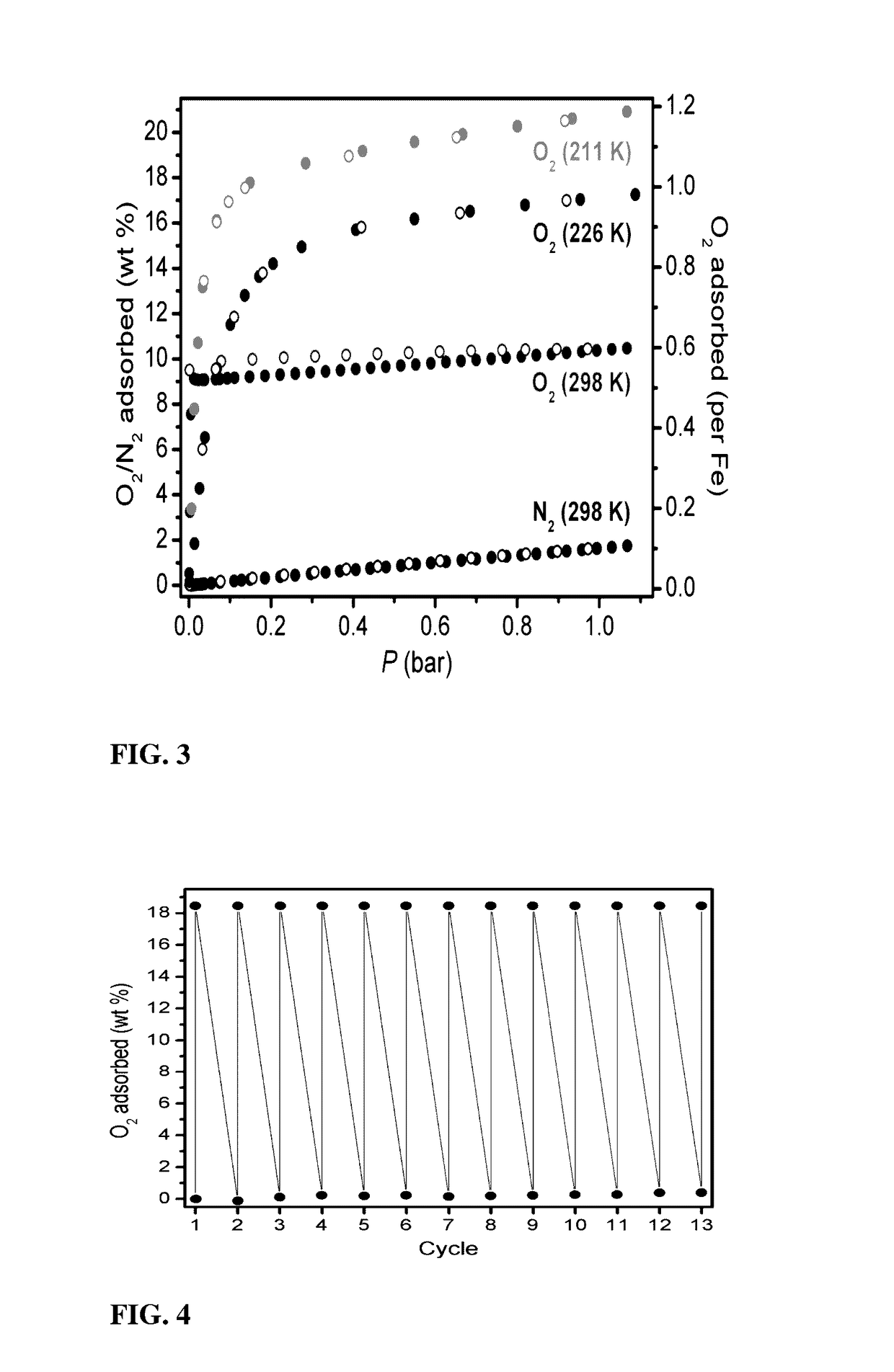
Fe2(dobdc) has a metal-organic framework with a high density of coordinatively-unsaturated FeII centers lining the pore surface. It can be effectively used to separate O2 from N2 and in a number of additional separation applications based on selective, reversible electron transfer reactions. In addition to being an effective O2 separation material, it can be used for many other processes, including paraffin / olefin separation, nitric oxide / nitrous oxide separation, acetylene storage, and as an oxidation catalyst.
Owner:RGT UNIV OF CALIFORNIA
Photoelectric converter, and transparent conductive substrate for the same
InactiveUS20100248418A1Improve photoelectric conversion efficiencyLowering of conversionLight-sensitive devicesSemiconductor/solid-state device manufacturingSemiconductor electrodeElectron transfer reactions
Owner:SONY CORP
Sulfonated-graphene-based novel Ru(bpy)3<2+> nano heterogeneous catalyst and preparation method thereof
InactiveCN103977835AEliminate the effects of diffusionUnique two-dimensional planar structure of single atomic layerCatalyst carriersOrganic compound preparationPhotoinduced electron transferPtru catalyst
The invention discloses a sulfonated-graphene-based novel Ru(bpy)3<2+> nano heterogeneous catalyst and a preparation method thereof. The nano heterogeneous catalyst comprises a sulfonated graphene matrix and Ru(bpy)3<2+> groups, wherein the sulfonated graphene matrix comprises a graphene matrix and sulfonic groups distributed on the graphene matrix; the Ru(bpy)3<2+> groups are connected onto the sulfonated graphene matrix after being matched with the sulfonic groups, and the active sites of the Ru(bpy)3<2+> groups are dispersed on a two-dimensional plane of the graphene matrix. The preparation process comprises the following steps: carrying out an ion exchange reaction on sulfonated reduction graphene and Ru(bpy)3<2+> to form the nano heterogeneous catalyst. The nano heterogeneous catalyst is high in chemical and heat stability, has catalytic active sites of Ru(bpy)3<2+> with visible-light activity, is high in catalytic activity, can be well dispersed in a reaction system, is easy to recover, is suitable for repeated use, and can be widely applied to a light-induced electron transfer reaction; simultaneously, the preparation process is simple, the raw material is low in cost and easily available, the cost is low and the need of large-scale production is met.
Owner:SHANGHAI NORMAL UNIVERSITY
Solid writing material
InactiveUS20150376433A1Improve light resistanceGood molding effectPencil leadsNon-propelling pencilsElectron transfer reactionsColor changes
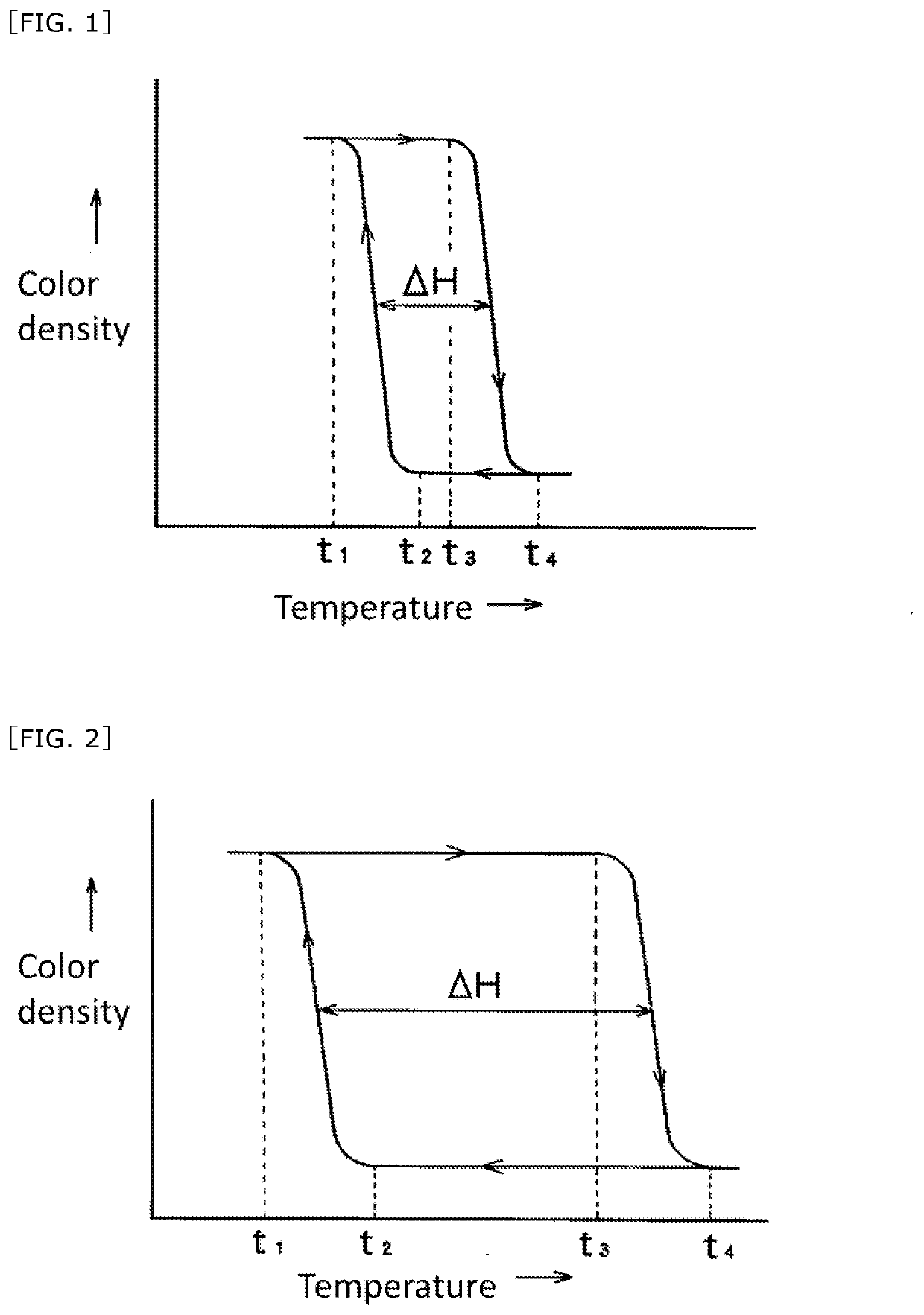
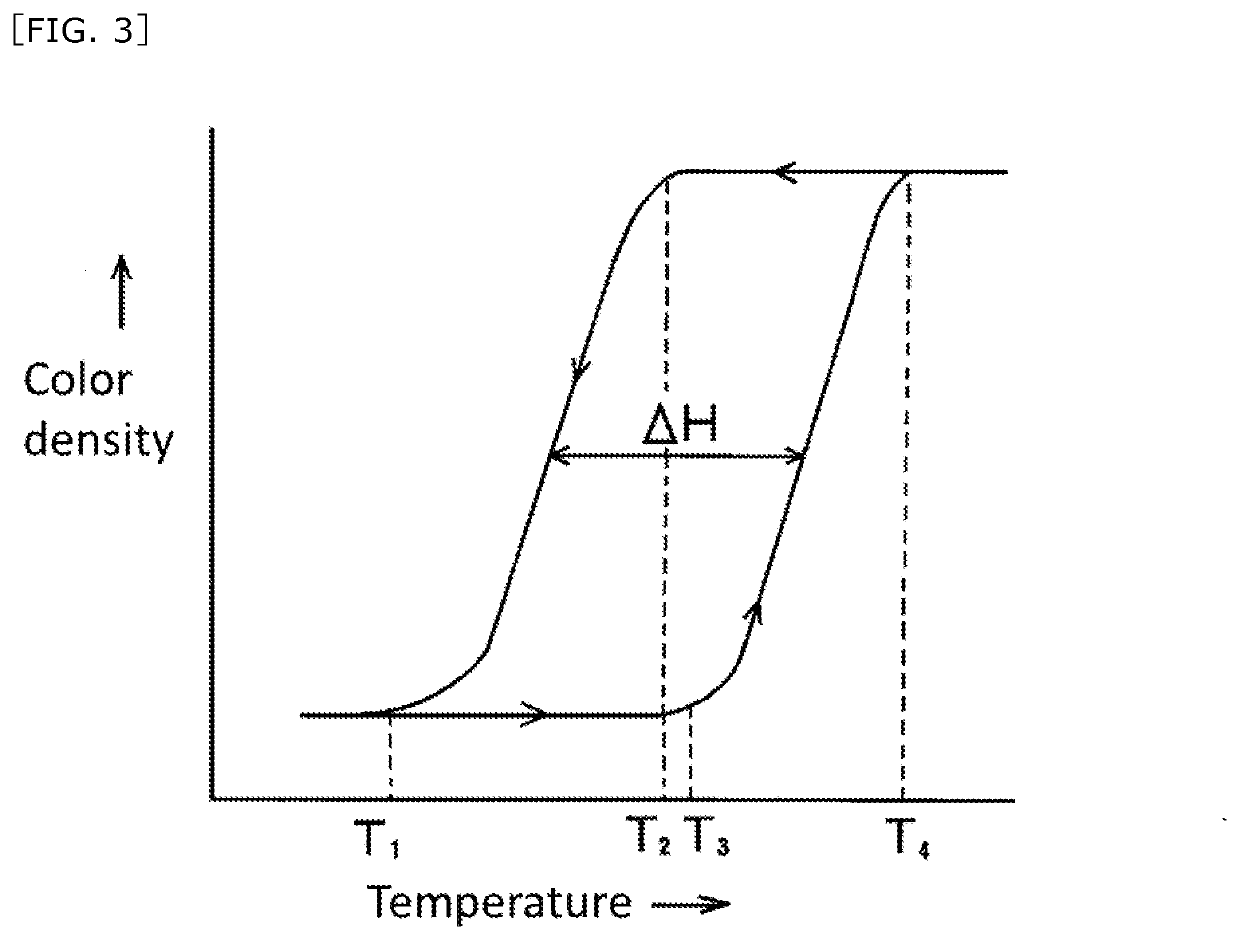
The invention aims at providing a solid writing material having a good color changing property or color extinguishing property, and having an excellent light resistance, moldability and strength. The solid writing material includes an inner core usable for writing and an outer shell covering an outer peripheral surface of the inner core; the inner core including a reversible thermochromic microcapsuled pigment encapsulating a temperature-sensitive color-changeable color-memorizing composition and an excipient; the temperature-sensitive color-changeable color-memorizing composition including at least (a) an electron-donating coloring organic compound, (b) an electron accepting compound and (c) a reaction medium effecting reversibly an electron transfer reaction between the (a) and (b) components in a specific temperature range; the outer shell including a filler.
Owner:PILOT PEN CO LTD
Application of porphyrin as catalyst in li/socl2 battery and battery
InactiveCN102263289AIncrease the open circuit voltageIncrease battery capacityOrganic-compounds/hydrides/coordination-complexes catalystsSecondary cellsSolubilityElectron transfer reactions
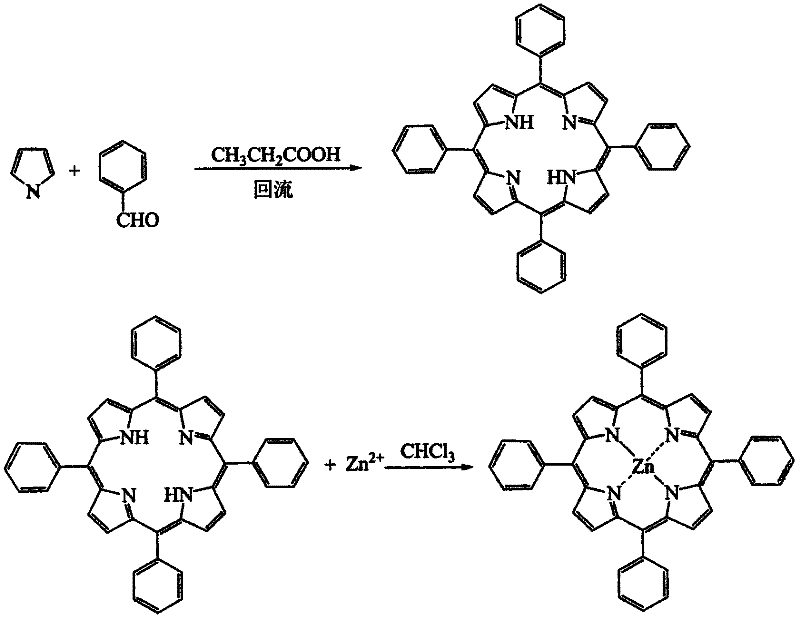
The invention discloses the application of porphyrin as a catalyst in Li / SOCl2 batteries. Both porphyrin and phthalocyanine are highly conjugated compounds containing macrocycles with similar catalytic properties. In thionyl chloride electrolyte, due to the influence of the polarity of the solution, the solubility of porphyrin is higher than that of phthalocyanine, which is beneficial to more More porphyrins participate in the catalytic reaction of the battery, which is beneficial to the transfer of electrons in the battery reaction. Using Zn(TPP) as a catalyst to catalyze Li / SOCl2 battery can effectively improve the open circuit voltage, battery capacity and average voltage of the battery. The solubility of porphyrin complexes in SOCl2 electrolyte is larger than that of phthalocyanine complexes, therefore, it is more beneficial for more metalloporphyrins to participate in the electron transfer reaction of the battery.
Owner:王君龙
Organic photovoltaic cells
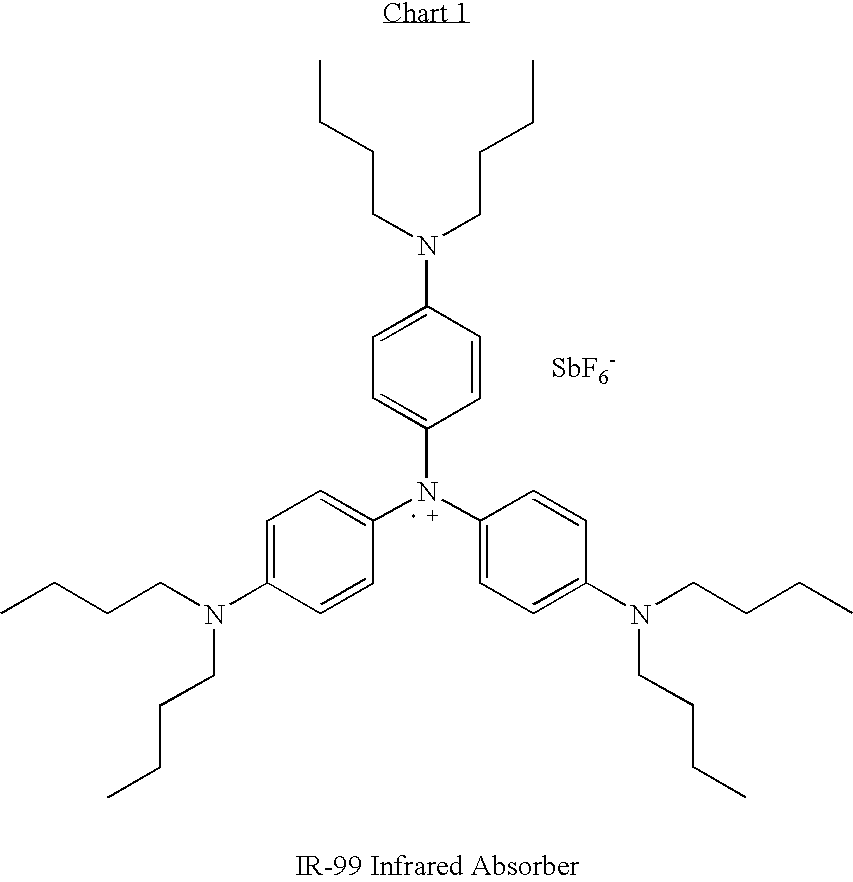
InactiveUS20080115833A1Solid-state devicesPhotovoltaic energy generationElectron transfer reactionsElectron
The present invention pertains to organic infrared absorbing layers comprising an organic radical cation compound that absorbs in an infrared region and produces an electron transfer reaction that results in a flow of electrons. Preferably, the organic radical cation compound is a salt of an aminium radical cation. Provided are photovoltaic cells comprising such light-sensitive organic infrared absorbing layers.
Owner:CARLSON STEVEN ALLEN
Copper oxide electrode material of nano-porous structure and preparation method and application thereof
InactiveCN106745180AEasy transferImprove electrochemical performanceNanotechnologyCopper oxides/halidesElectron transfer reactionsNew energy
The invention discloses a copper oxide electrode material of a nano-porous structure and a preparation method and application thereof, and belongs to the technical field of new energy and electrochemical sensor electrodes. The method comprises the steps of dropwise adding a copper acetate water solution into an oxalic acid water solution and reacting under a magnetic stirring condition; and carrying out cleaning and drying treatment by using deionized water and absolute ethyl alcohol and then burning to obtain the copper oxide electrode material of the nano-porous structure. The material is of a porous cystic three-dimensional structure, the front surface is a square, the edge is in a flat form, the middle is convex, and the side surface is in a spindle form. The material is dispersed into a nafion solution and coats a glassy carbon electrode which is polished through Al2O3 turbid liquid and treated through a sonic degradation method to obtain the electrochemical sensor electrode. Electron transfer reaction can be promoted, good electrochemical activity is provided, electron transmission is facilitated, and the copper oxide electrode material has potential application values.
Owner:YANGZHOU UNIV
Ink composition for reversibly thermochromic stamp and stamp
ActiveUS20170088737A1Reduce blurReduce unevennessInksStampingThermochromismElectron transfer reactions
An ink composition for a reversibly thermochromic stamp including a reversibly thermochromic microencapsulated pigment encapsulating a reversibly thermochromic composition including:Component (A): an electron-donating color-developing organic compound,Component (B): an electron-accepting compound, andComponent (C): a reaction medium causing reversibly an electron transfer reaction between the Component (A) and the Component (B) in a specific temperature range, water and a thickener.
Owner:PILOT PEN CO LTD +1
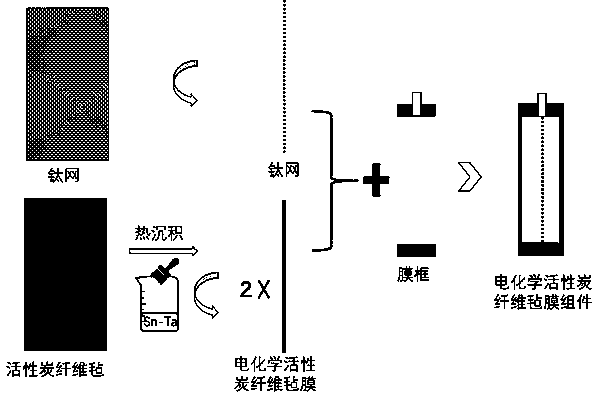
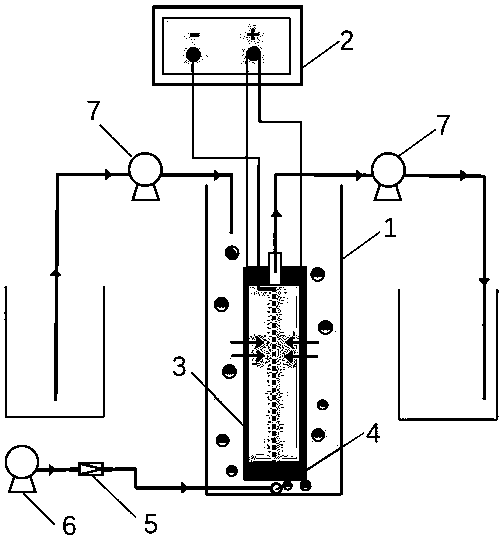
Electrochemical activated carbon fiber felt reactor applicable to removal of refractory organic matters in water
ActiveCN108658177AReduce pollutionHigh oxygen evolution potentialSemi-permeable membranesTreatment by combined electrochemical biological processesFiberElectron transfer reactions
The invention relates to an electrochemical activated carbon fiber felt reactor applicable to removal of refractory organic matters in water. A titanium mesh cathode is embedded into a PVC membrane bracket, ACFF / SnO2-Ta serves as an anode and a filter membrane at the same time, and an electrochemical microfiltration membrane assembly is placed in a reactor. The reactor is applied with an externalelectric field by a regulated DC power supply, and an oxidant variety with a relatively strong oxidizing property is produced in situ; in a continuous flow mode, convective mass transfer of a bulk solution and an anode interface is promoted by pumping of a peristaltic water outlet pump, so that an electron transfer reaction at the anode interface is accelerated, the yield of the oxidant is increased, and the probability of contact between organic pollutants in water and the produced oxidant is increased; particles, colloids and macromolecular pollutants in water can be filtered out through a dimensional exclusion effect of membrane pores, and because of relatively high specific surface area thereof, the electrochemical activated carbon fiber felt reactor can provide a large number of adsorption sites for small molecular organic pollutants in water; through doping of a Ta element, the electrocatalytic performance of SnO2 is significantly improved; H2O can form a large number of adsorbedand dissociated -OH through discharge at an anode, so that an important role is played in degradation of the small molecular organic pollutants.
Owner:TONGJI UNIV
High-rate long-service-life aqueous zinc-based battery based on double-electron reaction
InactiveCN112599864AAvoid structureHigh specific capacityFinal product manufactureCell electrodesElectrolytic agentSingle electron
The invention relates to a high-rate long-service-life aqueous zinc-based battery based on a double-electron reaction. According to the invention, an aqueous electrolyte of the battery is an electrolyte with organic carboxylic zinc / manganese salt as solute and also comprises a halogen-based ion enhanced additive; a positive electrode is manganese oxide, and a negative electrode is metal zinc or zinc alloy. During discharging, the manganese oxide of the positive electrode obtains electrons, is reduced into manganese ions and is dissolved in the electrolyte; and meanwhile, the zinc / zinc alloy ofthe negative electrode gives out electrons to generate zinc ions into the solution. During charging, manganese ions in a positive electrode region lose electrons, are oxidized into manganese oxide and are deposited on a positive electrode current collector, and zinc ions at the negative electrode obtain electrons and are reduced into metal zinc / zinc alloy. The charging and discharging processes are alternately carried out. The weakly-acidic reaction system of the aqueous zinc-based battery provided by the invention has the beneficial effects that the weakly-acidic reaction system utilizes thedouble-electron transfer reaction of manganese oxide, and compared with a traditional single-electron transfer reaction, specific capacity is doubled, and higher energy density is obtained.
Owner:浙江浙能中科储能科技有限公司 +2
Ozone modified beta radioactive ion source and application thereof
InactiveCN103165390ARealize online modificationRealize switchingMaterial analysis by electric/magnetic meansIon sources/gunsHalogenElectron transfer reactions
The invention relates to an ion source in an analytical instrument, in particular to a beta radioactive ion source modified by ozone. A part of O2 in clean air is firstly translated into O3 through an ozone generating device and then enters the beta radioactive ion source, and O2-(H2O)n reagent ions generated by the clean air in the beta radioactive ion source are reacted with O3 to generate new reagent ions O3-(H2O)n. The new generated reagent ions can not only generate electron transfer reaction with electronegative compounds (such as explosive some substances and halogen compounds), but also generate O- transfer reaction with some components (such as SO2 and CO2), so that number of the channels of compound ionizing in the beta radioactive ion source are increased, and the enlargement of detectable range of the compounds can be benefited.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Solid writing material
ActiveUS20160075904A1Good effectImprove impact resistanceOrganic chemistryPencil leadsElectron transfer reactionsColor changes
The invention aims at providing a solid writing material that does not adversely affect the color changing property of script and can obtain sufficient strength. This aim is achieved by the solid writing material that includes: a reversible thermochromic microcapsuled pigment encapsulating a temperature-sensitive color-changeable color-memorizing composition; an excipient; and a resin; the temperature-sensitive color-changeable color-memorizing composition including at least (a) an electron-donating coloring organic compound, (b) an electron accepting compound and (c) a reaction medium effecting reversibly an electron transfer reaction between the (a) and (b) components in a specific temperature range; wherein the excipient or the resin includes a styrene-structure-containing polymer.
Owner:PILOT PEN CO LTD
Dye sensitization solar cell based on synergetic catalytic binary redox couple
InactiveCN103093960AImprove performanceInhibitory complexLight-sensitive devicesFinal product manufactureElectron transfer reactionsSolar cell
The invention discloses a dye sensitization solar cell based on synergetic catalytic binary redox couple. The dye sensitization solar cell comprises a substrate, a transparent electrode, a metallic oxide photo-anode, dyes, a counter electrode and an electrolyte which is capsulated between the photo-anode and the counter electrode. The electrolyte contains mutually synergetic catalytic binary redox couple , such as organic compounds which contain hydrosulphonyl and organic P-type semiconductor. Two kinds of materials which form the binary redox couple are coupled on the molecule level, so that redox reaction with mutual catalytic reaction can be achieved, and electron transfer reaction inside the solar cell is facilitated. Therefore, recombination between a photoproduction electron and a hole can be restrained and device performance can finally be improved.
Owner:PEKING UNIV
Preparation method of manganese dioxide/carbon nanotube complex fuel cell cathode oxygen reduction catalyst
InactiveCN109346734ALow source costThe control method is simple and convenientMaterial nanotechnologyCell electrodesElectron transfer reactionsCarbon nanotube
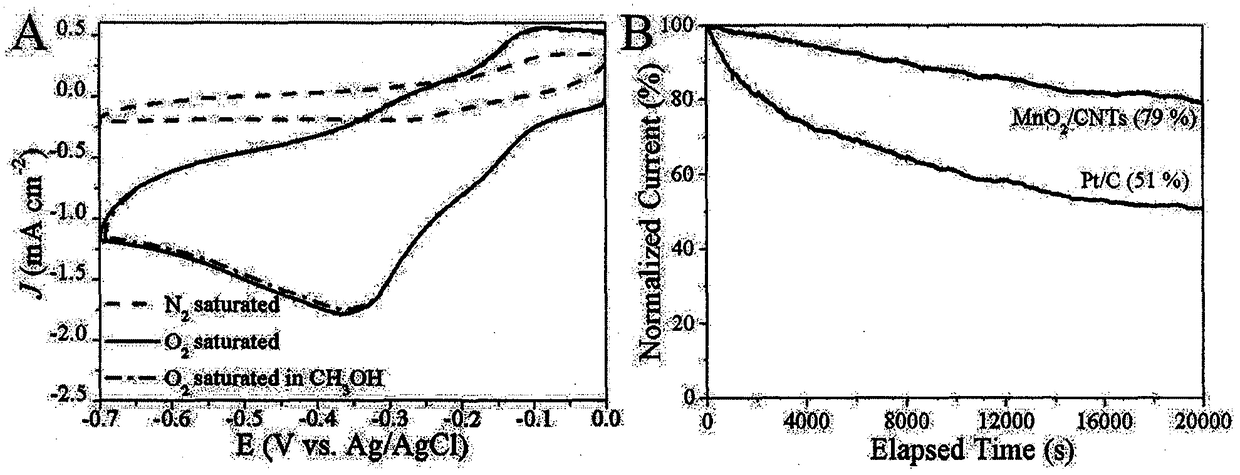
The invention discloses a preparation method of a manganese dioxide / carbon nanotube complex fuel cell cathode oxygen reduction catalyst and belongs to the field of energy. The preparation method comprises the following steps: firstly activating a carbon nanotube, then uniformly mixing the carbon nanotube with potassium permanganate, performing hydrothermal reaction in a high-pressure reactor and finally calcining under argon to obtain a manganese dioxide / carbon nanotube complex. The complex as a precious metal-free catalyst is applied to oxygen reduction reaction in an alkaline medium, shows excellent catalytic activity and shows the near-four electron transfer reaction. The manganese dioxide / carbon nanotube complex also has stronger methanol resistance and superior long range stability. Due to low cost and wide adaptability, the manganese dioxide / carbon nanotube complex is one of potential materials for the fuel cell cathode catalyst and is expected to replace a precious metal catalyst. The preparation method disclosed by the invention has the benefits that the process is simple, the operation is easy, and a synthetic material has an ordered mesoporous structure, more active sitesand excellent catalytic property.
Owner:YANCHENG TEACHERS UNIV
Method of making alkali and gypsum by proton-coupled electron transfer reaction
InactiveUS20210047742A1Difficult to purifyReduce contentElectrolysis componentsCarbonate purificationPtru catalystElectron transfer reactions
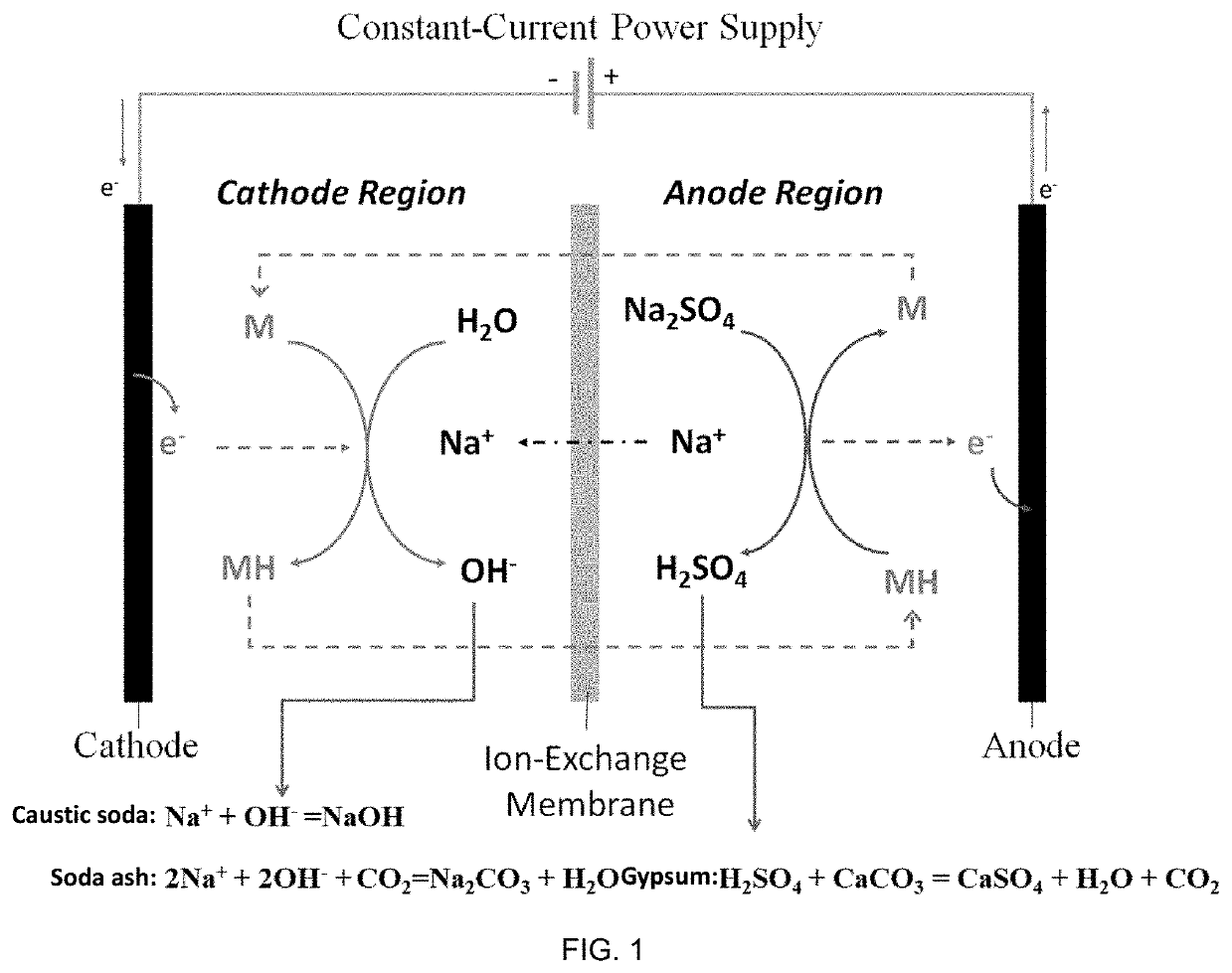
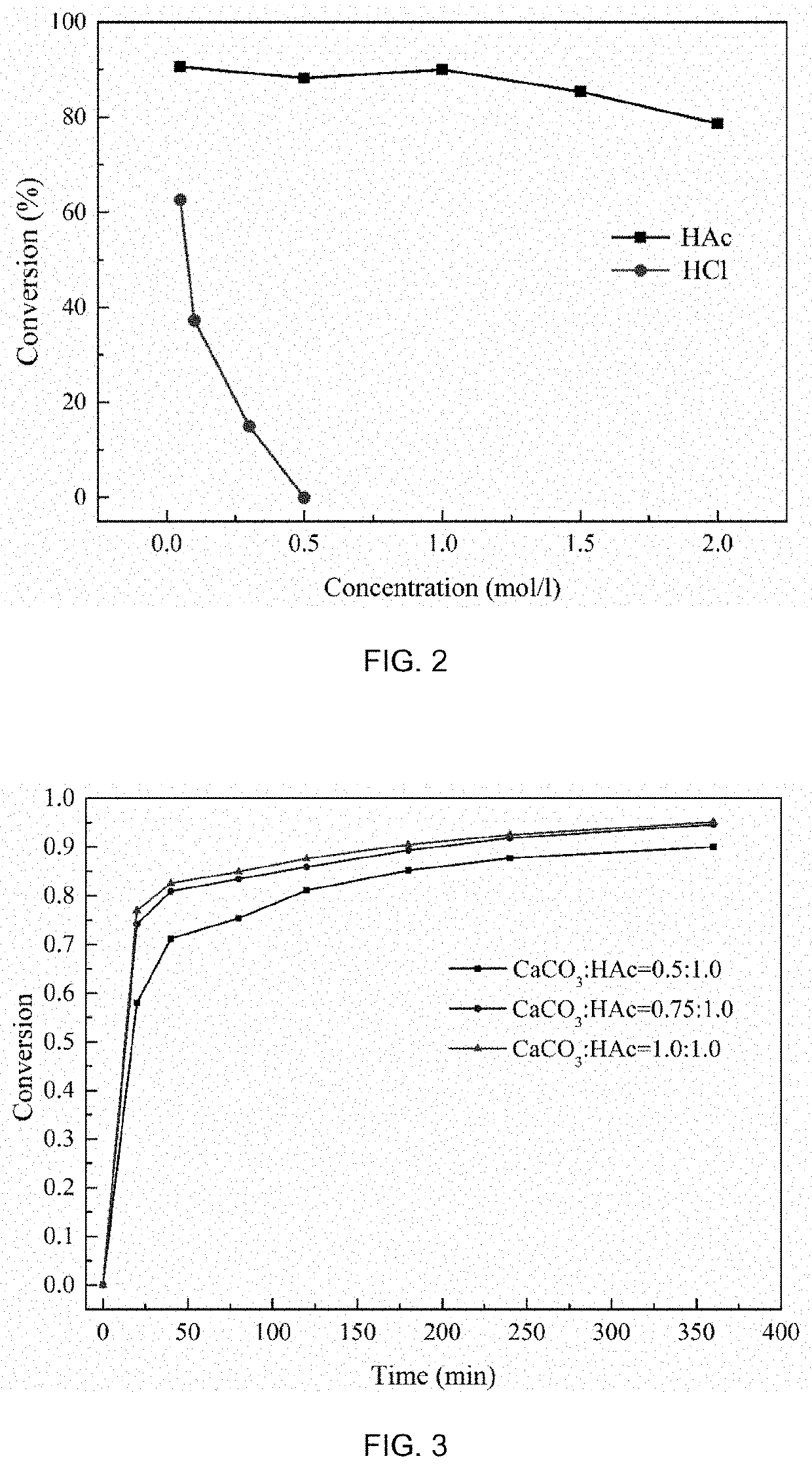
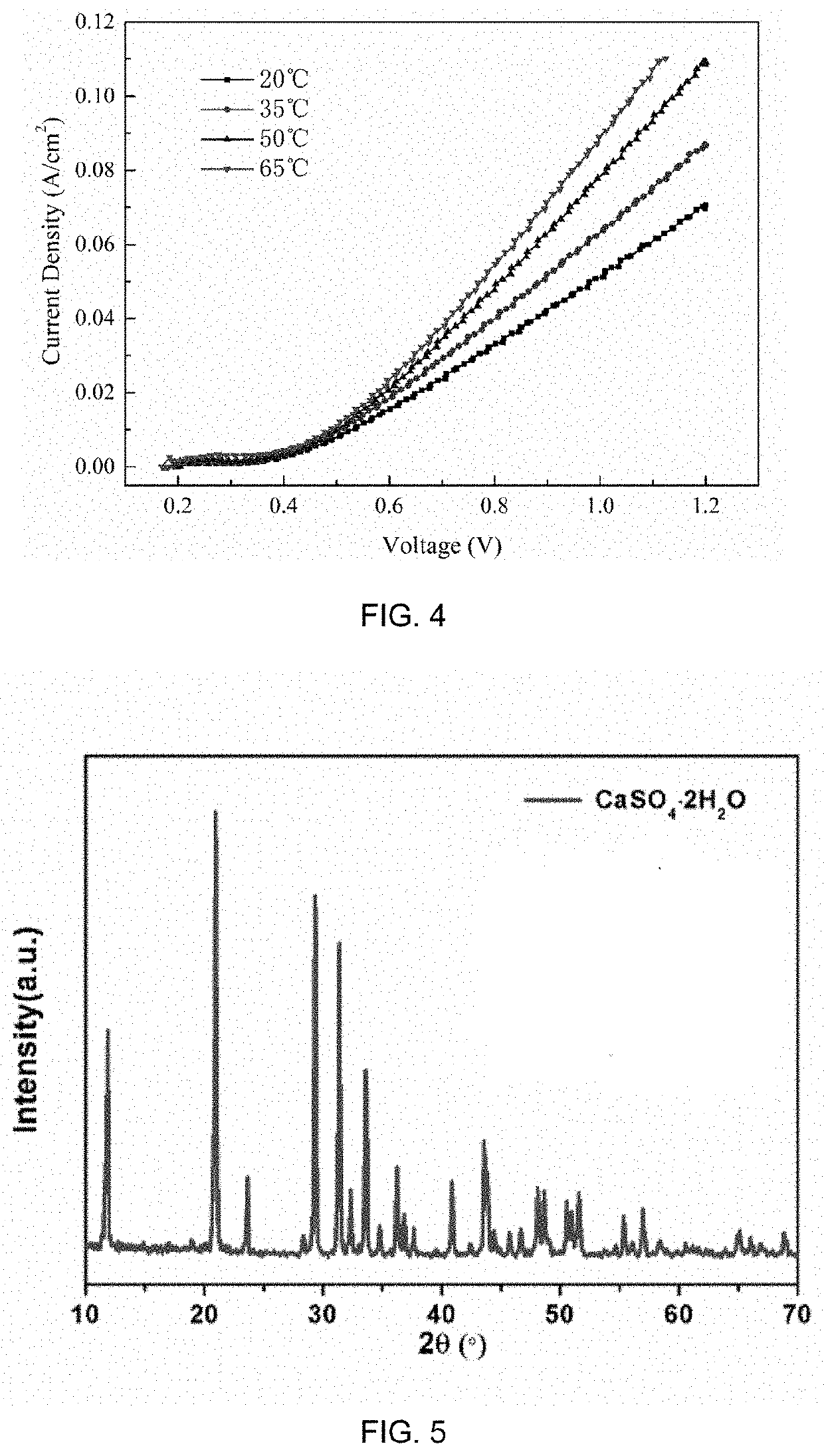
The present disclosure provides a method for preparing an alkali and co-producing gypsum, and belongs to the technical field of chemical production. The method comprises the steps of placing a cation exchange membrane into an electrolytic cell, adding a solution of sodium salt of a weak acid and a compound MH to an anode region as an anode electrocatalyst, adding sodium carbonate or sodium hydroxide to a cathode region, adding a compound M as a cathode electrocatalyst, and applying a DC power supply between a cathode electrode and an anode electrode. The electrolysis oxidizes the MH into the M and releases H+, Na+ in the anolyte penetrates through the cation exchange membrane to reach a cathode region to be combined with OH− in the catholyte to generate NaOH, or further absorbs CO2 and converts into Na2CO3; the anolyte containing a large amount of H+ is generated by the electrolysis for dissolution reaction with limestone, and the H+ is consumed to generate Ca2+, and SO42− and Ca2+ are combined to generate high-purity CaSO4 precipitate. According to the present disclosure, a compound capable of generating PCET reaction is used as an electrocatalyst, while M is its oxidation state and MH is its reduction state, and mirabilite and limestone are used as raw materials to realize the preparation of soda ash, caustic soda and gypsum.
Owner:WANG YUFEI +1
Enzyme electrode, and bio fuel cell equipped therewith
ActiveUS20130059212A1Increase productionCell electrodesMicrobiological testing/measurementFuel cellsElectron transfer reactions
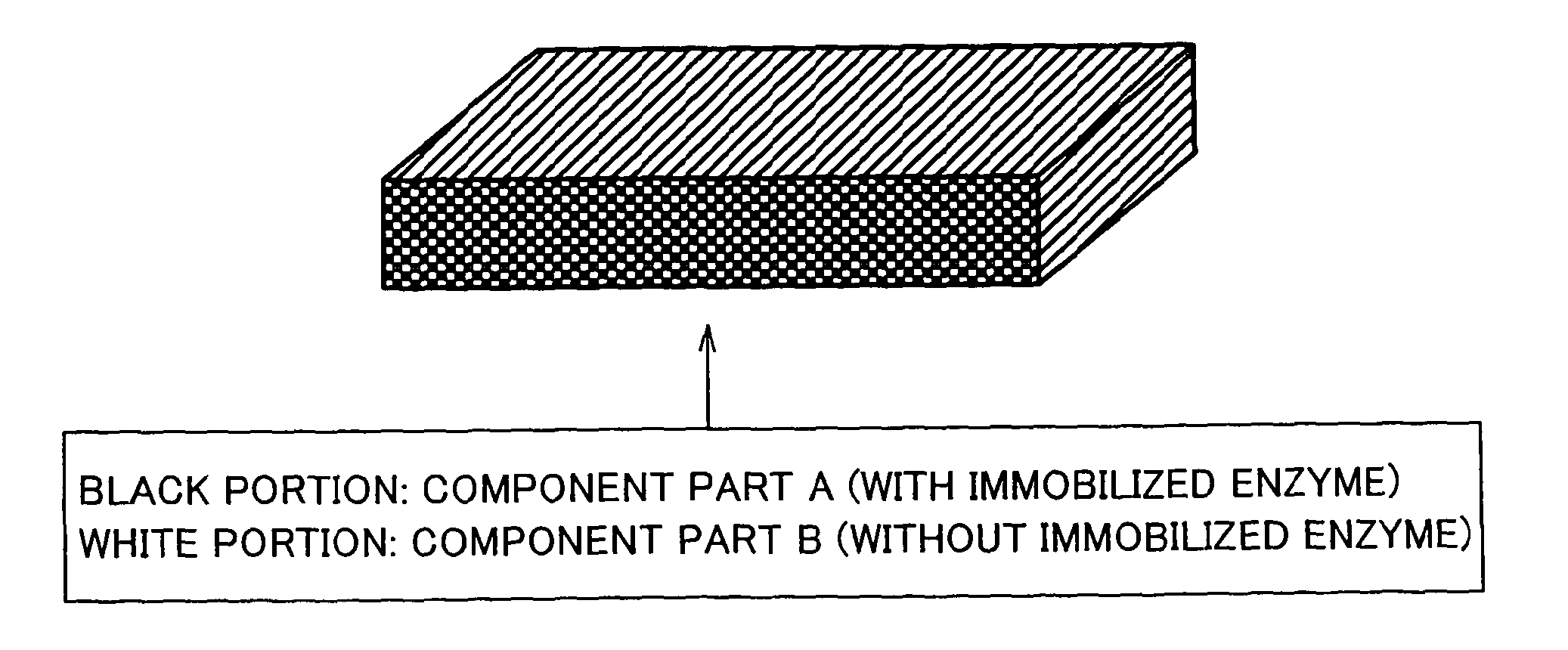
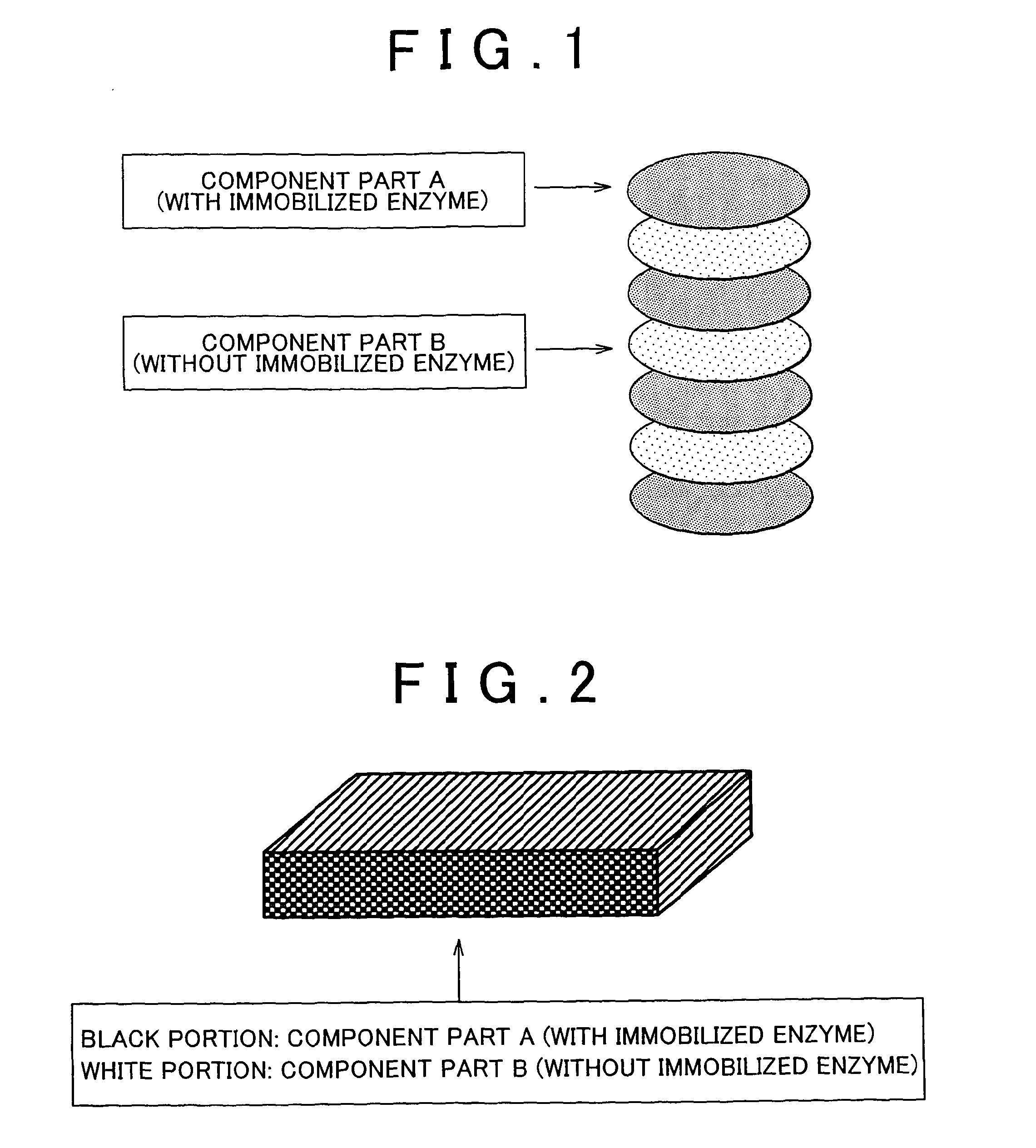
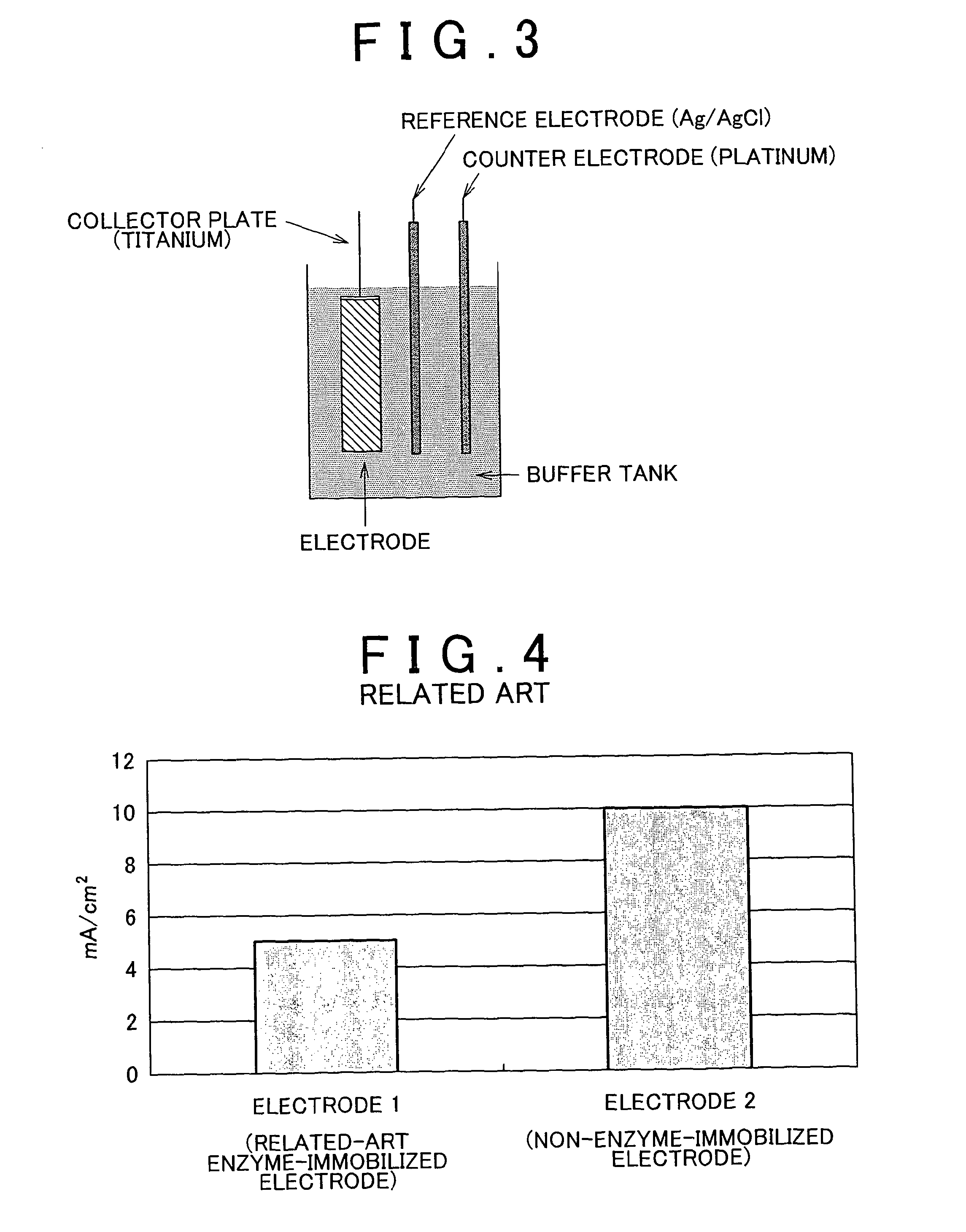
An enzyme electrode having an electroconductive base member, an oxidoreductase and an electron mediator has at least a portion (a) in which the oxidoreductase is immobilized on the electroconductive base member, and a portion (b) in which the electron mediator is immobilized on the electroconductive base member but the oxidoreductase is not immobilized on the electroconductive base member. A bio fuel cell having the enzyme electrode as at least one of an anode and a cathode allows optimization of a reaction condition of each one of a plurality of reaction steps, including an “enzymatic reaction”, an “electron transfer reaction”, etc. Thus, the bio fuel cell provides high output.
Owner:TOYOTA JIDOSHA KK
Surface plasmon resonance detection with high angular resolution and fast response time
InactiveCN1216282CImprove signal-to-noise ratioStrong specificityScattering properties measurementsMaterial analysis by electric/magnetic meansElectron transfer reactionsPrism
A device and method of detecting surface plasmon resonance for sensing molecules or conformational changes in molecules with high resolution and fast response time is disclosed. Light from a light source (14) is focused through a prism onto a metal thin film (15) on which sample molecules to be detected are adsorbed. The total internal reflection of the laser / incident light is collected with a differential position or intensity sensitive photo-detecting device instead of a single cell or an array of photo-detectors (12) that are widely used in previous works. The ratio of the differential signal to the sum signal of the differential position or intensity sensitive photo-detecting device (12) provides an accurate measurement of the shift in the surface plasmon resonance angle caused by the adsorption of molecules onto the metal films (15) or by conformational changes in the adsorbed molecules. The present invention requires no numerical fitting to determine the resonant angle and the setup is compact and immune to background light, The methods and sensors of this invention can be used in numerous biological, biochemical, and chemical applications such as measuring subtle conformational changes in molecules and electron transfer reactions can be studied.
Owner:佛罗里达国际大学董事会
Organic electroluminescent device, display substrate and display device
ActiveCN109768178ASolid-state devicesSemiconductor/solid-state device manufacturingElectron transfer reactionsDisplay device
The invention provides an organic electroluminescent device, a display substrate and a display device and belongs to the field of display technology. The organic electroluminescent device can solve the problem that an existing OLED has relatively low efficiency, relatively high driving voltage and relatively high power consumption. In the organic electroluminescent device provided by the invention, a P type doping material of a hole transport layer does not need a deep LUMO energy level, hole carrier injection on the hole transport layer is realized by utilizing an electron transfer reaction performed on the P type doping material when the P type doping material is excited by lights, anode contact resistance is effectively reduced, hole injection is increased, voltage is reduced, efficiency of the organic electroluminescent device is improved, and power consumption is reduced.
Owner:BOE TECH GRP CO LTD +1
Reversibly thermochromic composition and reversibly thermochromic microcapsule pigment encapsulating the same
ActiveUS20200048542A1Improve light resistanceVivid colorsInksThermographySimple Organic CompoundsThermochromism
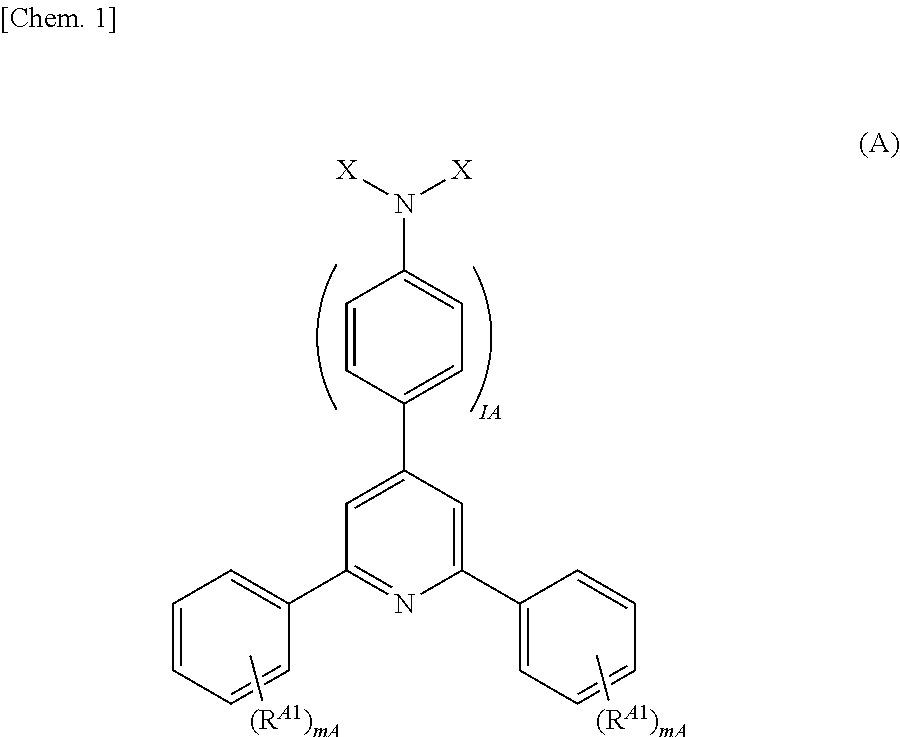
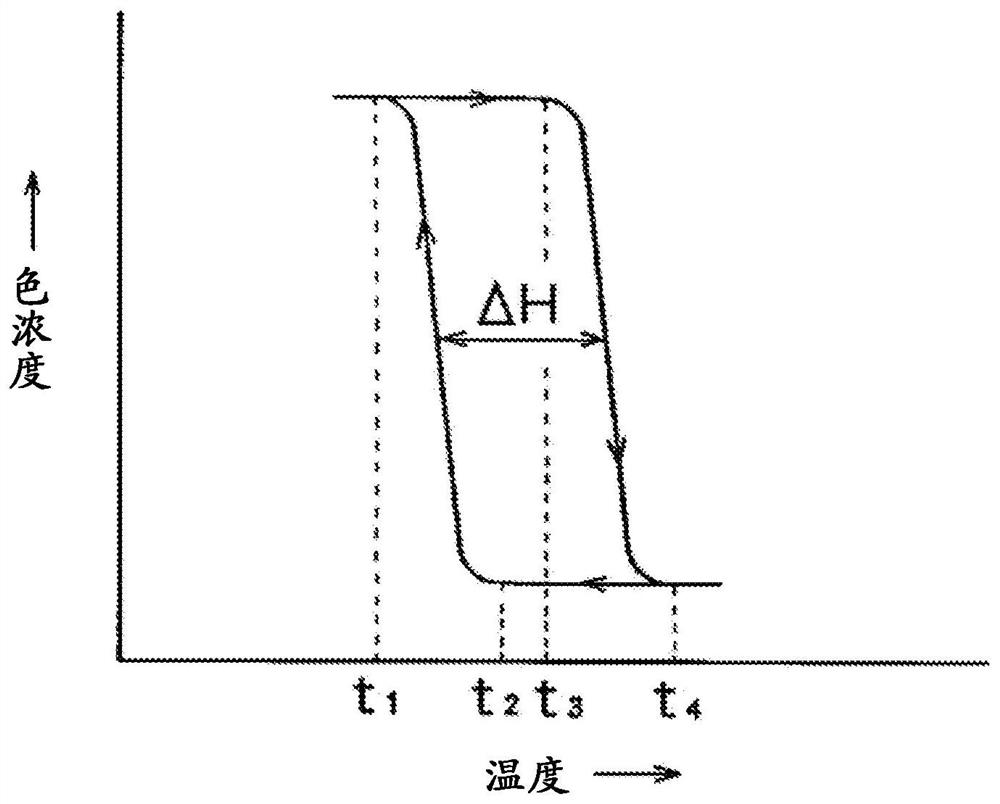
[Problems] The present invention provides a highly marketable reversibly color changeable composition which provides a vivid yellow to orange color during color development, turns colorless during decoloration, and improves light resistance and a reversibly thermochromic microcapsule pigment encapsulating the same.[Solution] Disclosed are a reversibly thermochromic composition including: (a) a specific pyridine derivative as an electron-donating color-developing organic compound; (b) an electron-accepting compound; and (c) a reaction medium which reversibly induces an electron transfer reaction between the component (a) and the component (b) in a specific temperature range, and a reversibly thermochromic microcapsule pigment encapsulating the same.
Owner:PILOT PEN CO LTD
Durable enzyme-based biosensor and process for drop deposition immobilization
ActiveCN110023745AAntifouling/underwater paintsMaterial analysis by electric/magnetic meansAmmonium compoundsElectron transfer reactions
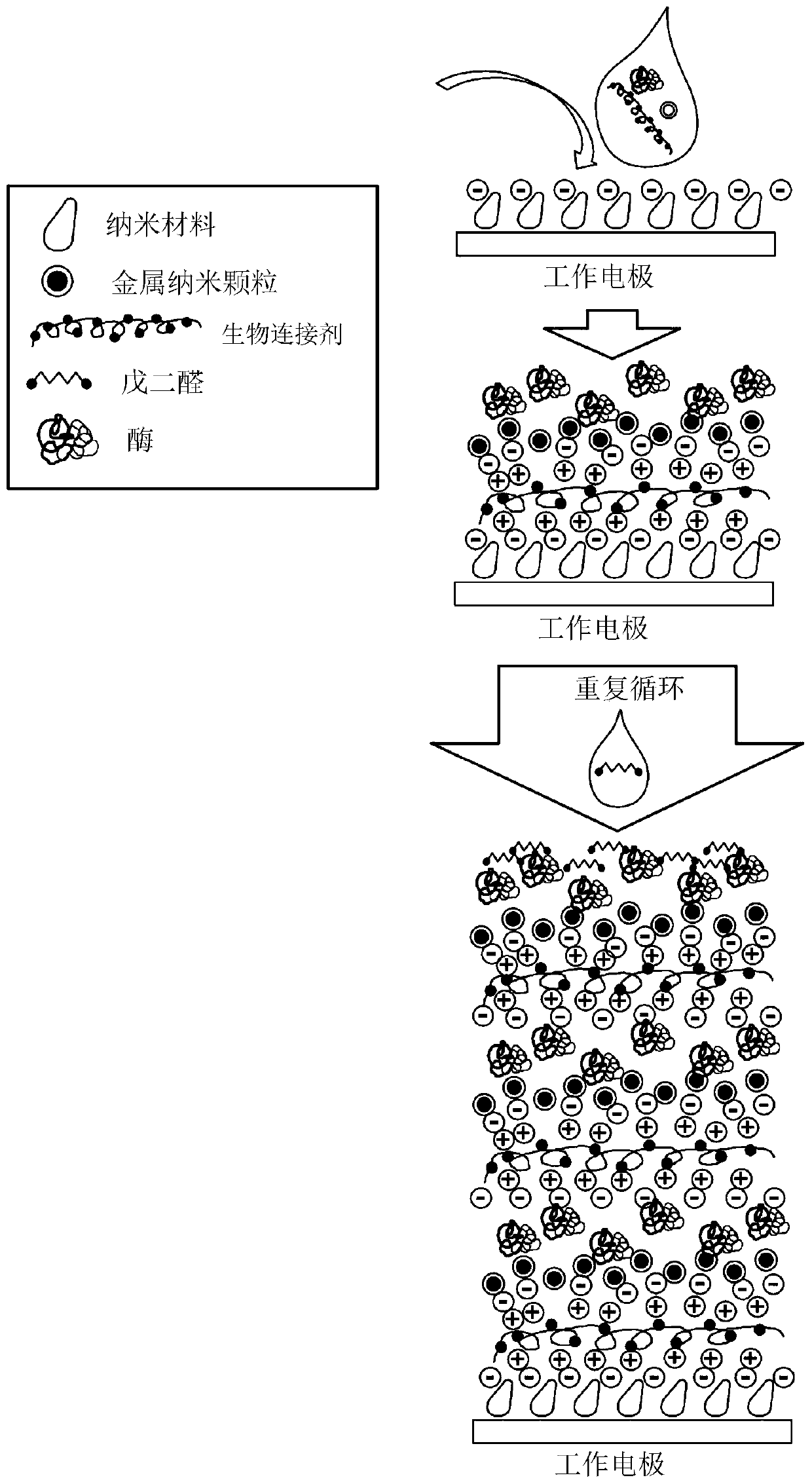
A sensor having improved shelf life is provided for determining concentration of a biomarker in a liquid sample. The sensor functions by electrochemical detection and requires use of a bio-molecule, e.g., an enzyme that catalyzes an electron transfer reaction specific for the biomarker. The sensor requires use of a quaternary ammonium compound as a bio-linker. It further requires crosslinking of the bio-molecule for improved stability.
Owner:NORTHEASTERN UNIV
Reversible thermochromic composition, reversible thermochromic microcapsule pigment comprising same, and writing instrument using same
PendingCN113646052ACosmetic preparationsPyronine/xanthon/thioxanthon/selenoxanthan/telluroxanthan dyesElectron transfer reactionsPhotopigment
[Problem] To provide a reversible thermochromic composition, and a microcapsule pigment comprising the same, the reversible thermochromic composition: exhibiting a black color at the time of color development and color changing to colorless when decolorized; having excellent contrast between a color-developed state and a decolorized state; and having a high commercial value, by using a fluoran derivative having a specific structure as an electron-donating coloring organic compound. [Solution] Provided is a reversible thermochromic composition comprising: (a) a fluoran derivative being an electron-donating and coloring organic compound and having a specific structure; (b) an electron accepting compound; and (c) a reaction medium that reversibly causes an electron transfer reaction by means of component (a) and component (b) in a specific temperature range. Also provided is a reversible thermochromic microcapsule pigment comprising the reversible thermochromic composition.
Owner:THE PILOT INK CO LTD +1
Gas separations with redox-active metal-organic frameworks
ActiveUS9675923B2Nitrous oxide captureOxygen-containing compound preparationHigh densityElectron transfer reactions
Fe2(dobdc) has a metal-organic framework with a high density of coordinatively-unsaturated FeII centers lining the pore surface. It can be effectively used to separate O2 from N2 and in a number of additional separation applications based on selective, reversible electron transfer reactions. In addition to being an effective O2 separation material, it can be used for many other processes, including paraffin / olefin separation, nitric oxide / nitrous oxide separation, acetylene storage, and as an oxidation catalyst.
Owner:RGT UNIV OF CALIFORNIA
Monomolecular fluorescence resonance energy transfer method based on photo-activation
ActiveCN106918584AIncreased maximum concentration limitBreaking through the concentration barrierFluorescence/phosphorescenceChemical reactionElectron transfer reactions
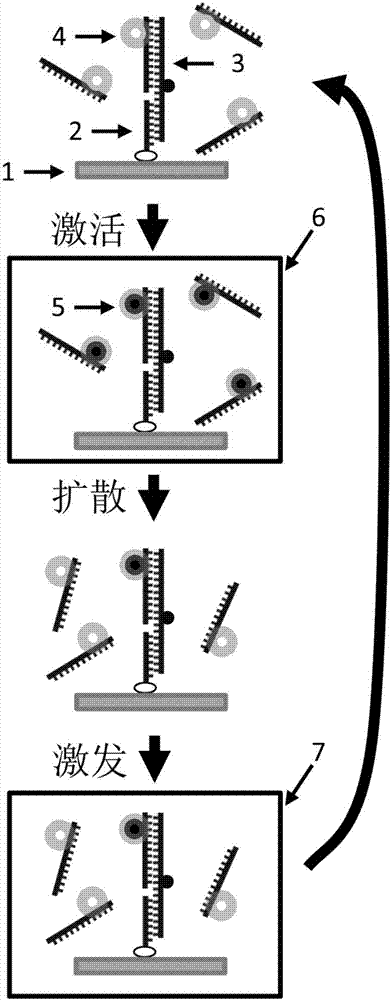
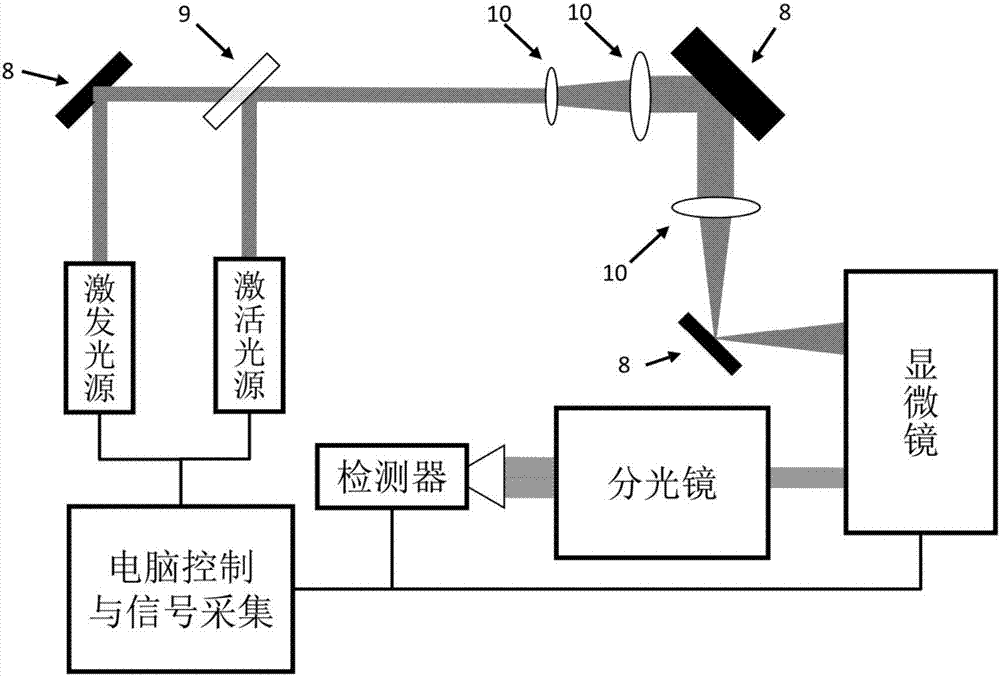
The invention discloses a monomolecular fluorescence resonance energy transfer method based on photo-activation, and belongs to the technical field of biological macromolecule imaging. A photo-activated fluorescent dye is used to label a biological sample taken as the donor. An activation light source with a wavelength of 200 to 450 nanometers is used to trigger the photo-activated fluorescent dye to carry out chemical reactions such as cis-trans isomerism reactions, electron transfer reactions, chemical bond breaking reactions, and the like. The photo-activated fluorescent dye is converted from a non-activated state that does not emit fluorescence into an activated state that can emit fluorescence. Then an excitation light source with a wavelength of 450 to 1200 nanometers is used to excite the fluorescent dye so as to emit fluorescence. When the distance between a donor and an acceptor labeled by a non-photo-activated fluorescence dye is in a range of 1-10 nanometers, the energy of the donor is transferred to the acceptor, namely fluorescence resonance energy transfer. The method has the advantages that in a conventional fluorescence resonance energy transfer technology, the concentration of a fluorescence sample should reach about 50 nM, while in the provided method the measurement of monomolecular fluorescence resonance energy transfer can be carried out under a micro-mole concentration, which is close to the physiological conditions.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com