High-rate long-service-life aqueous zinc-based battery based on double-electron reaction
A zinc-based battery, high-rate technology, applied in battery electrodes, secondary batteries, secondary battery repair/maintenance, etc., can solve the problems of manganese dioxide structure collapse, reaction limitations, etc. The effect of specific capacity improvement and high rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] An aqueous zinc-based secondary battery based on a two-electron reaction, the negative electrode is zinc foil, and the positive electrode is graphite felt containing electrodeposited manganese (IV) oxide.
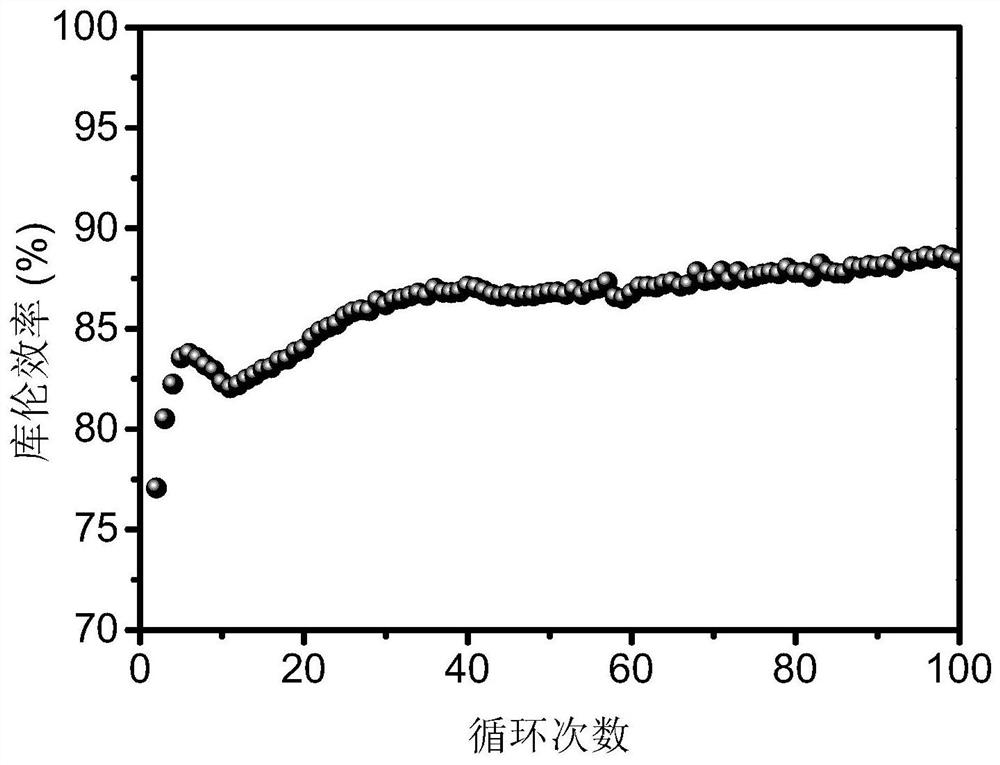
[0029] The organic carboxylic acid zinc salt / manganese salt electrolyte contains 1 mol / L zinc acetate and 0.5 mol / L manganese acetate. 1.8V constant voltage charging to 0.5mAh / cm 2 , followed by 5mA / cm 2 Constant current discharge to 1V under current density. The battery test results are attached figure 1 As shown, the Coulombic efficiency of the first cycle is 68.35%, but after 100 cycles, the Coulombic efficiency is stable at 88.37%, indicating that the two-electron reaction aqueous zinc-based secondary battery has good reversibility and cycle stability.
Embodiment 2
[0031] An aqueous zinc-based secondary battery based on a two-electron reaction, the negative electrode current collector is zinc foil, and the positive electrode is graphite felt containing electrodeposited manganese (IV) oxide.
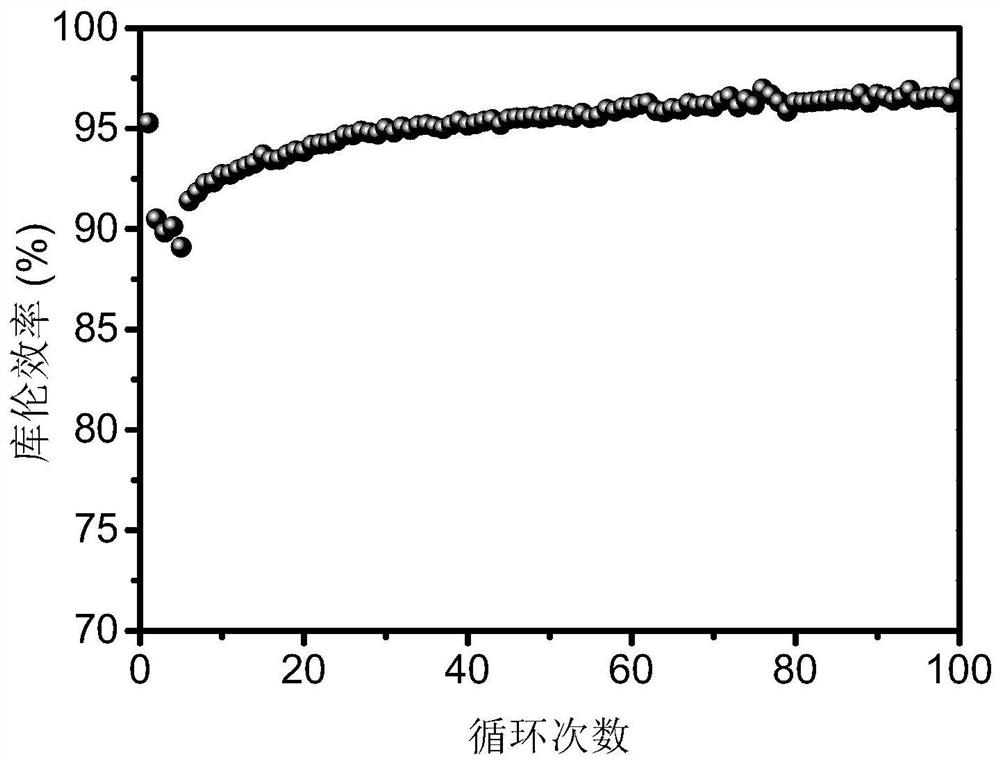
[0032] The organic carboxylic acid zinc salt / manganese salt electrolyte contains 1 mol / L zinc acetate, 0.5 mol / L manganese acetate, and 0.3 mol / L acetic acid. 1.8V constant voltage charging to 0.5mAh / cm 2 , then at 5mA / cm 2 Constant current discharge to 1V under current density. The battery test results are attached figure 2 As shown, the Coulombic efficiency in the first cycle is 95.28%, and the Coulombic efficiency stabilizes at 97.04% after 100 cycles. Therefore, the introduction of organic carboxylic acid into the electrolyte can improve the Coulombic efficiency and cycle life of the two-electron reaction aqueous zinc-based secondary battery.
Embodiment 3
[0034] An aqueous zinc-based secondary battery based on a two-electron reaction, the negative electrode current collector is zinc foil, and the positive electrode is graphite felt containing electrodeposited manganese (IV) oxide.
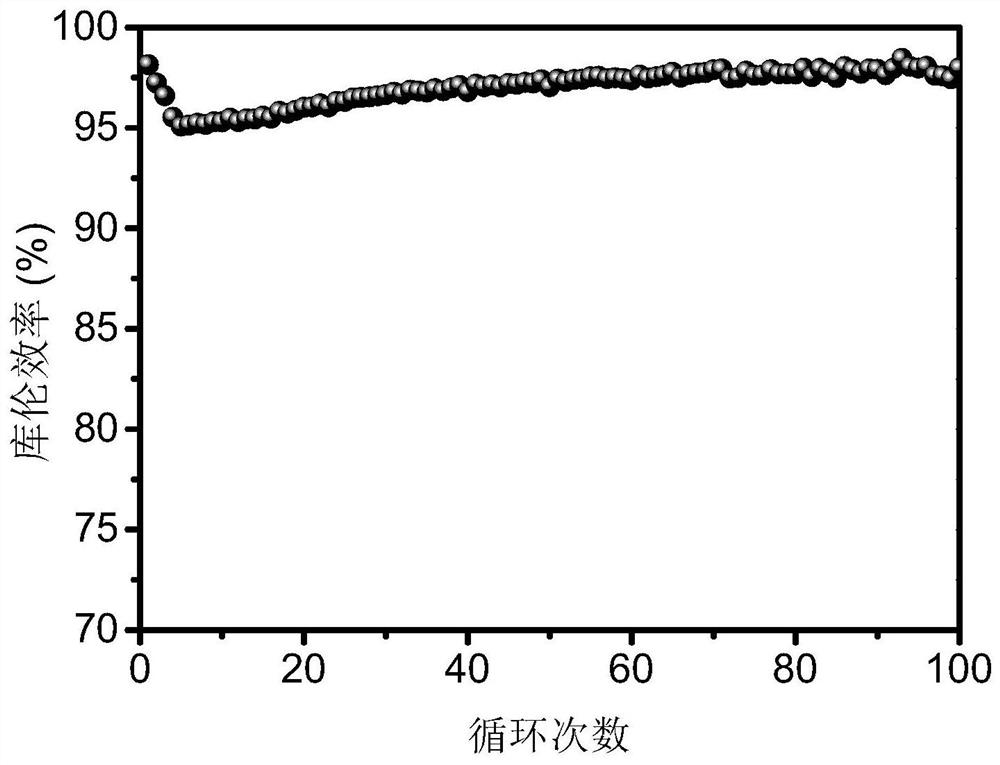
[0035] The electrolyte solution contains 1 mol / L zinc acetate, 0.5 mol / L manganese acetate, and 0.5 mol / L acetic acid. 1.8V constant voltage charging to 0.5mAh / cm 2 , then at 5mA / cm 2 Constant current discharge to 1V under current density. The battery test results are attached image 3 As shown, the Coulombic efficiency of the first cycle is 98.15%, and the Coulombic efficiency is stable at 97.99% after 100 cycles. Therefore, increasing the concentration of organic carboxylic acid can improve the coulombic efficiency and cycle life of the two-electron reaction aqueous zinc-based secondary battery.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com