Transformation method and application of structurally rearranged derivatives of diterpene-type compounds of caperia
A technology for compounds and diterpenes, applied in the field of structural rearrangement derivatives, can solve the problems that have not yet been seen, and achieve the effects of improving druggability and enhancing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
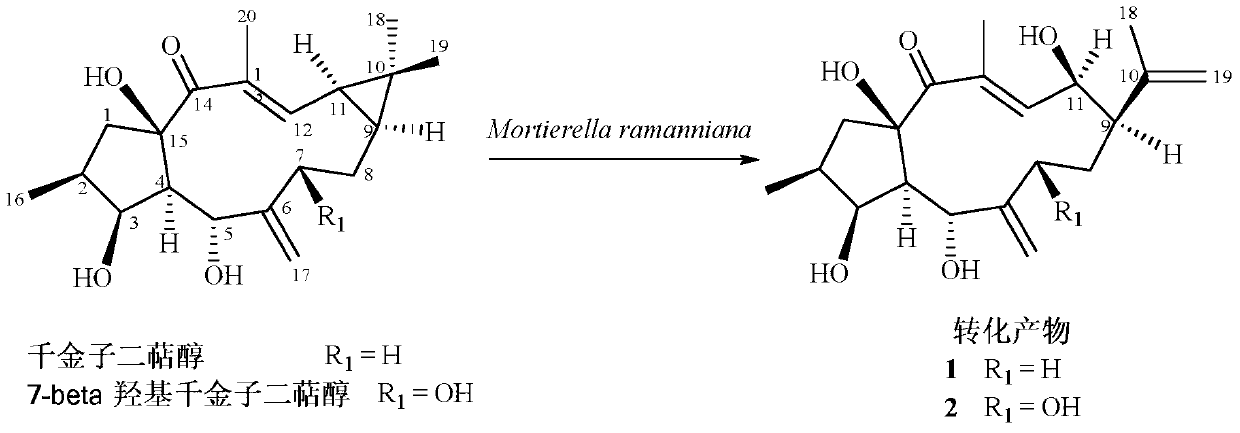
[0027] Example 1 High Performance Liquid Chromatography (HPLC) Examination of Mortierella ramanniana Transforming Lathyrol and 7β-Hydroxystephanol into Rearranged Products
[0028] The strain screening medium is a potato medium: cut 200g of peeled potatoes into small cubes of 1 cubic centimeter, boil them with 1L of water for 20 minutes, add 20g of glucose after filtering the potato liquid, and distribute them in 250mL Erlenmeyer flasks, 50mL per bottle , sterilized at 121°C, 0.15Mpa for 20 minutes;
[0029] The strains were inoculated on the slant solid medium, cultured at 28°C for 7 days, and stored in a refrigerator at 4°C. The strains were activated by a two-step activation method. First, the strains were inoculated on the potato medium, and the flask was shaken at 28°C and 180rpm. After culturing for 48 hours, obtain the seed solution; inoculate the seed solution in another potato medium at a volume ratio of 1%-3%, and cultivate under the same conditions for 48 hours, and...
Embodiment 2
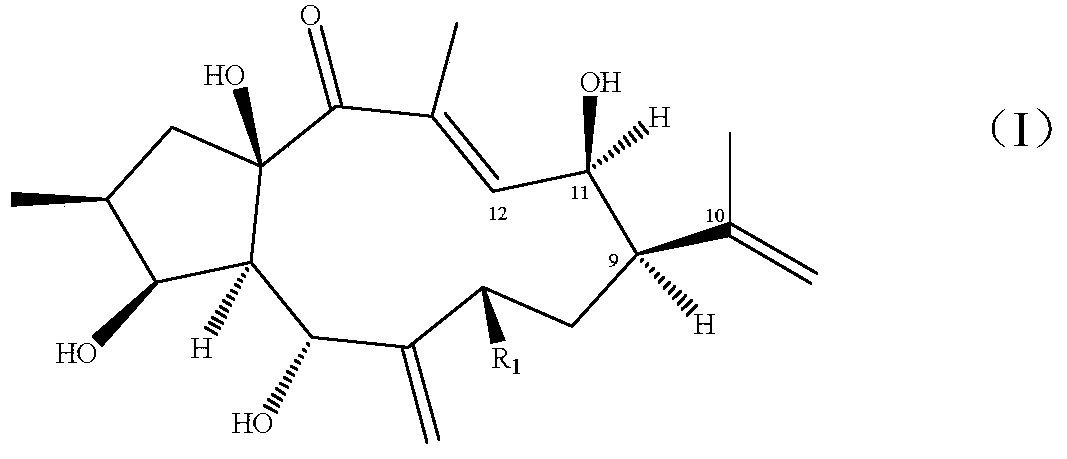
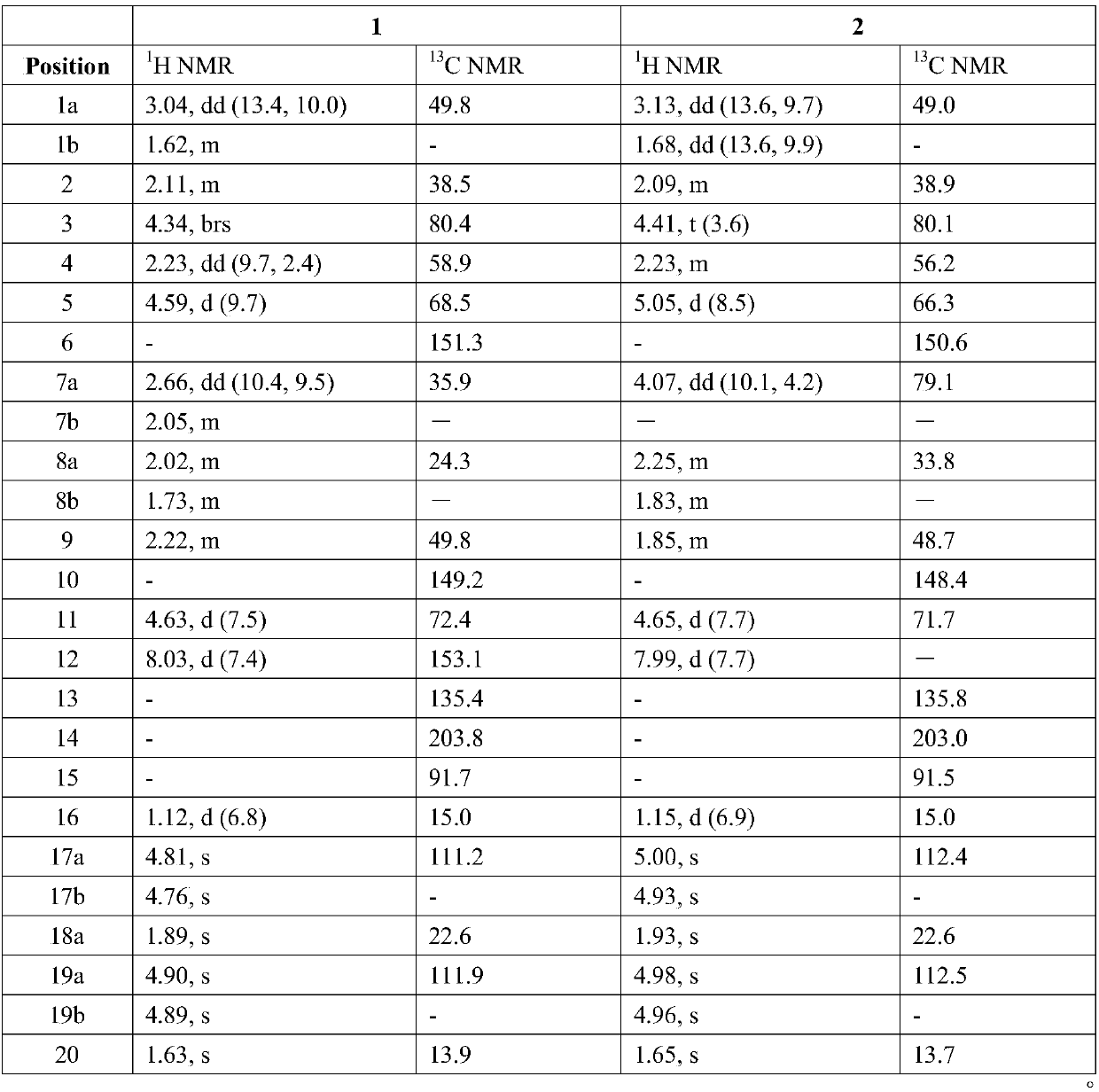
[0035] Embodiment 2 prepares the structural rearrangement product (1) of diterpene alcohol
[0036]The two-step activation method was used to activate the strains, and the obtained seed solution was inoculated into 250mL potato medium with a volume ratio of 1L in a 1L Erlenmeyer flask at a volume ratio of 1%, and was shaken at 28°C for 48h under the condition of 180rpm. Add 5mg / mL ethanol solution of stephenae diterpene alcohol to the bacterial solution, the final concentration is 0.1mg / mL, after 108 hours of cultivation under the same conditions, the fermentation broth is suction-filtered, the filtrate is extracted with ethyl acetate for 3 times, and ethyl acetate is recovered after combination , to obtain the total extract of the fermentation broth;
[0037] The total extract of the fermentation broth was dissolved with a small amount of ethyl acetate, mixed with 1g of silica gel, and wet-loaded with dichloromethane on a silica gel column equipped with 40g of column chromato...
Embodiment 3
[0038] Example 3 Preparation of Structural Rearrangement Product (2) of 7β-Hydroxystephania Diterpene Alcohol
[0039] The two-step activation method was used to activate the strains, and the obtained seed solution was inoculated into 250mL potato medium with a volume ratio of 1L in a 1L Erlenmeyer flask at a volume ratio of 1%, and was shaken at 28°C for 48h under the condition of 180rpm. Add 5 mg / mL ethanol solution of 7β-hydroxystephinol diterpene alcohol to the bacterial liquid, the final concentration is 0.1 mg / mL, after 108 hours of culture under the same conditions, the fermentation liquid is suction filtered, and the filtrate is extracted with ethyl acetate for 3 times, combined and recovered Ethyl acetate to obtain the total extract of the fermentation broth;
[0040] The total extract of the fermentation broth was dissolved in a small amount of ethyl acetate, mixed with 1g of silica gel, wet loaded with dichloromethane into a silica gel column equipped with 40g of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com