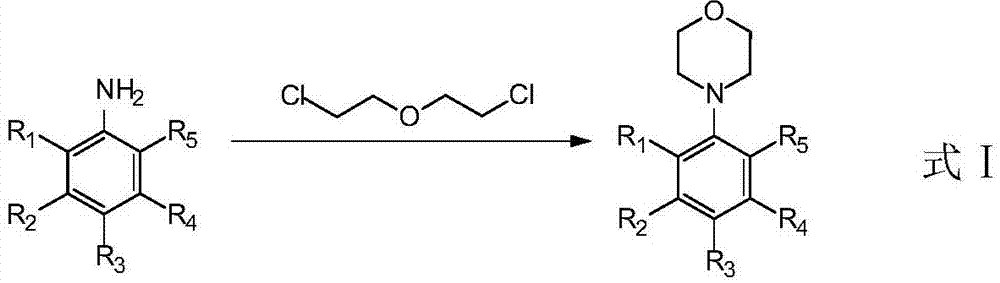
Method for synthesizing substitution N-phenylmorpholine compounds
A technology of phenylmorpholine and synthesis method, which is applied in the field of cyclic chiral amino compounds can be enlarged, can solve the problems of high cost, low yield, complicated operation and the like, and achieve the effects of cost reduction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
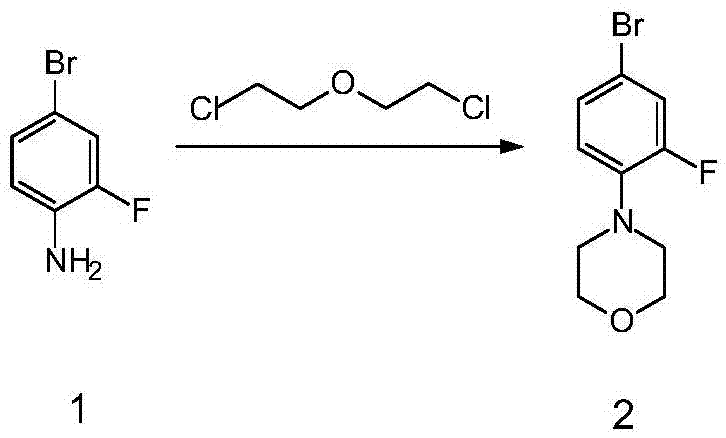
[0024] Example 1 Synthesis of 4-(2-fluoro-4-bromophenyl)morpholine
[0025]
[0026] Compound 1 (50g, 0.263mol), 500mL 2-chloroethyl ether, and triethylamine (53.3g, 0.526mol) were mixed, heated to 150°C and reacted for 24 hours. After the reaction was detected by TLC, the recovered solvent 2 was distilled off under reduced pressure. -Chloroethyl ether, cooled and poured into 1L of water, added EA (300ml×2) for extraction, dried with 20g of anhydrous sodium sulfate, decolorized with 5g of activated carbon, filtered and concentrated to obtain a off-white solid and spin-dried to obtain compound 2 (55g, yield: 80.4%)().
[0027] 1 H NMR (300MHz, CDCl 3 ): 7.20-7.23(m,2H), 6.80-6.84(t,1H), 3.87-4.0(t,4H), 3.06-3.09(t,4H).
Embodiment 2
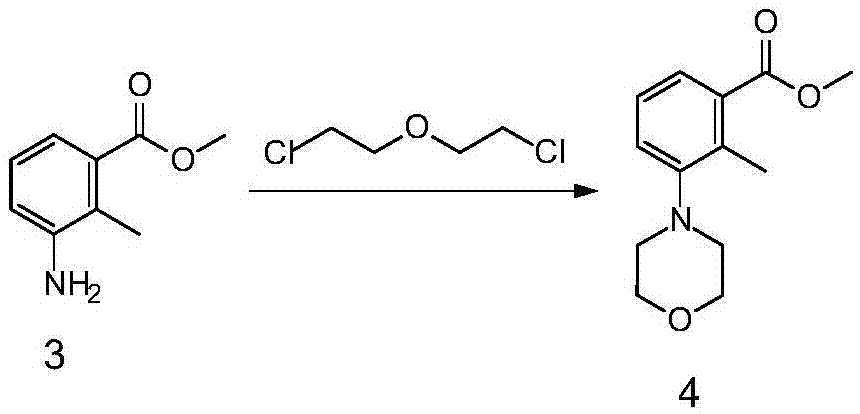
[0028] Example 2 Synthesis of 2-methyl-3-(4-morpholinyl) methyl benzoate
[0029]
[0030] Compound 3 (148.7g, 0.900mol), 700mL 2-chloroethyl ether, and triethylamine (228g, 2.25mol) were mixed and heated to 150°C for 4 hours. After the reaction was detected by TLC, the recovered solvent 2 was distilled off under reduced pressure. -Chloroethyl ether, cooled and poured into 1L water, added EA (500ml×2) for extraction, dried with 50g of anhydrous sodium sulfate, decolorized with 15g of activated carbon, filtered and concentrated to give off-white solid spin-dried to give compound 4 (140.4g, yield : 66.3%).
[0031] 1 H NMR (300MHz, CDCl 3 ):7.55-7.59 (d, 1H), 7.21-7.25 (m, 2H), 3.86-3.91 (m, 7H), 2.90-2.939 (t, 4H), 2.53 (s, 3H).
Embodiment 3
[0032] Example 3 Synthesis of 4-(2-methyl-4-bromophenyl)morpholine
[0033]
[0034] Compound 5 (100g, 0.537mol), 600mL 2-chloroethyl ether, and triethylamine (108.3g, 1.074mol) were mixed, and heated to 150°C for 24 hours. After the reaction was detected by TLC, the recovered solvent 2 was distilled off under reduced pressure. -Chloroethyl ether, cooled and poured into 1L of water, added EA (500ml×2) for extraction, dried with 50g of anhydrous sodium sulfate, decolorized with 15g of activated carbon, filtered and concentrated to give an off-white solid and spin-dried to obtain compound 6 (85g, yield: 61.7%).
[0035] 1 H NMR (300MHz, CDCl 3 ):7.30-7.32(m,2H),6.87-6.90(d,1H),7.61(m,2H),3.83-3.87(t,4H),2.85-2.89(t,4H),2.29(s,3H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com