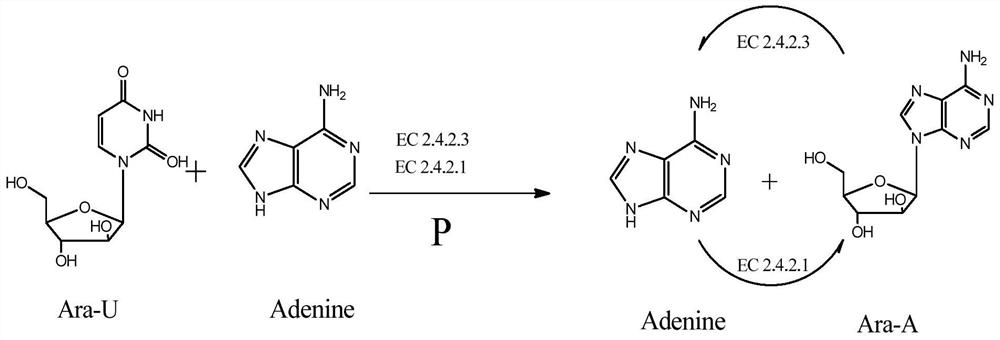
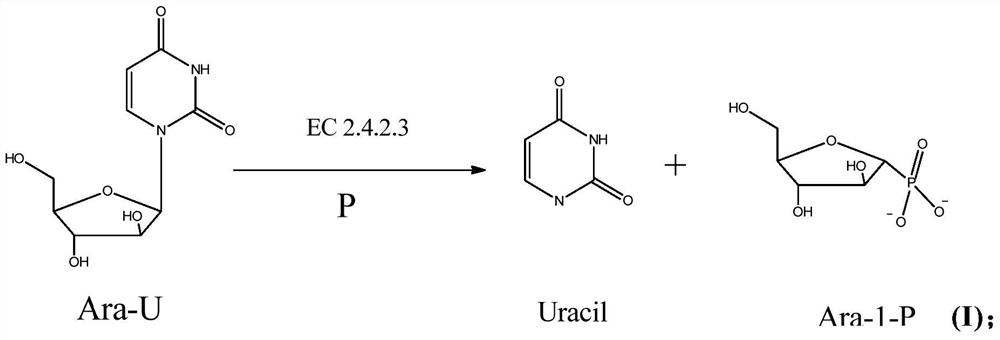
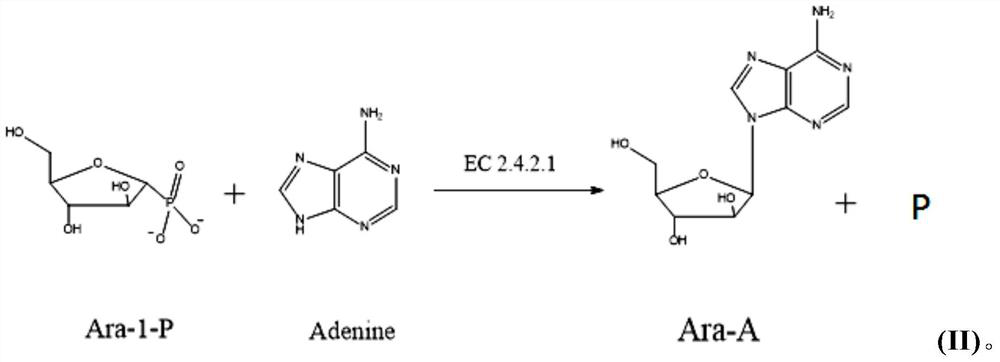
A method for enzymatically synthesizing vidarabine
A technology for vidarabine and vidarabine, which is applied in the field of enzymatic synthesis of vidarabine, can solve the problems of chemical synthesis pollution, large substrate consumption, low conversion rate of target substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090]Example 1, Two-step reaction 1
[0091]The reaction is carried out in two steps, as follows:
[0092]Response 1:
[0093]Add 0.04 mole of arabinouridine to 1000ml of 300mM sodium phosphate solution of pH6.8 to make the concentration reach 40mM, and then add 1.56g of bacteria (32200U per gram containing uridine phosphorylase) to make the concentration of uridine phosphorylase reach 50U / ml; this mixture is placed in a shaker and shaken at 55°C for 8 hours.
[0094]Take out the reaction solution and cool it to 10°C, add 34g of anhydrous calcium chloride solid, and stir for 30 minutes, ultrafiltration to remove the precipitated uracil, calcium phosphate and other insolubles, the insolubles include bacteria containing uridine phosphorylase Keep the ultrafiltration solution at 5-10℃, collect the ultrafiltration solution, wash the membrane with 500ml of double distilled water after completion, wash out the remaining reaction liquid remaining in the membrane bag, combine the ultrafiltration liqui...
Embodiment 2
[0099]Example 2. Two-step reaction 2
[0100]The reaction is carried out in two steps, as follows:
[0101]Response 1:
[0102]Add 0.2 mole of arabinouridine to 1000ml pH6.8 600mM sodium phosphate solution to make the concentration reach 200mM, and then add 6.21g bacteria (containing uridine phosphorylase 32200U per gram) to make the concentration of uridine phosphorylase reach 200U / ml; this mixture is placed in a shaker and shaken at 55°C for 8 hours.
[0103]Take out the reaction solution and cool it to 10°C, add 1000ml of double distilled water to dilute the reaction solution; add 68g of anhydrous calcium chloride solid, and stir for 30 minutes, ultrafiltration to remove the precipitated uracil, calcium phosphate and other insoluble matter, the insoluble Substances include bacteria containing uridine phosphorylase; keep the ultrafiltration solution at 5-10°C, wash the membrane with 500ml double-distilled water after completion, wash out the remaining reaction solution remaining in the membra...
Embodiment 3
[0108]Example 3, Comparative Example 1
[0109]Using the same method in Example 1, the main difference is that in "Reaction 1", after "shaking at 55°C for 8 hours", adenine is directly added for reaction 2, excluding the low-temperature ultrafiltration and "Reaction Two "in the step of double distilled water dilution.
[0110]The reaction is carried out in two steps, as follows:
[0111]Response 1:
[0112]Add 0.04 mole of arabinouridine to 1000ml of 300mM sodium phosphate solution of pH6.8 to make the concentration reach 40mM, and then add 1.56g of bacteria (32200U per gram containing uridine phosphorylase) to make the concentration of uridine phosphorylase reach 50U / ml; this mixture is placed in a shaker and shaken at 55°C for 8 hours.
[0113]The above-obtained mixture was diluted 3 times and then subjected to quantitative analysis by high performance liquid phase (ELSD). The peak area of arabin-1-phosphate was 72974.04. Substituting this number into the standard curve formula was calculated:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com