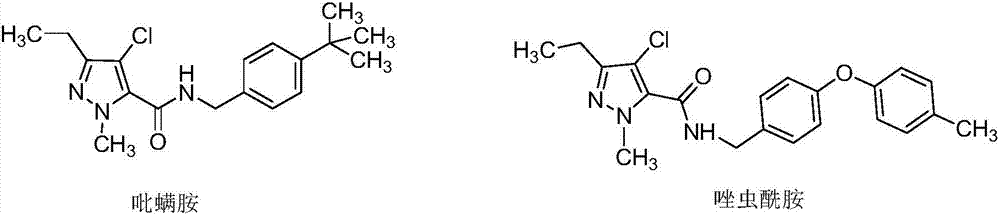
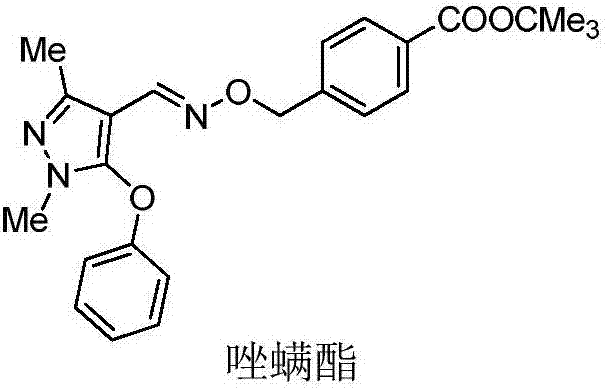
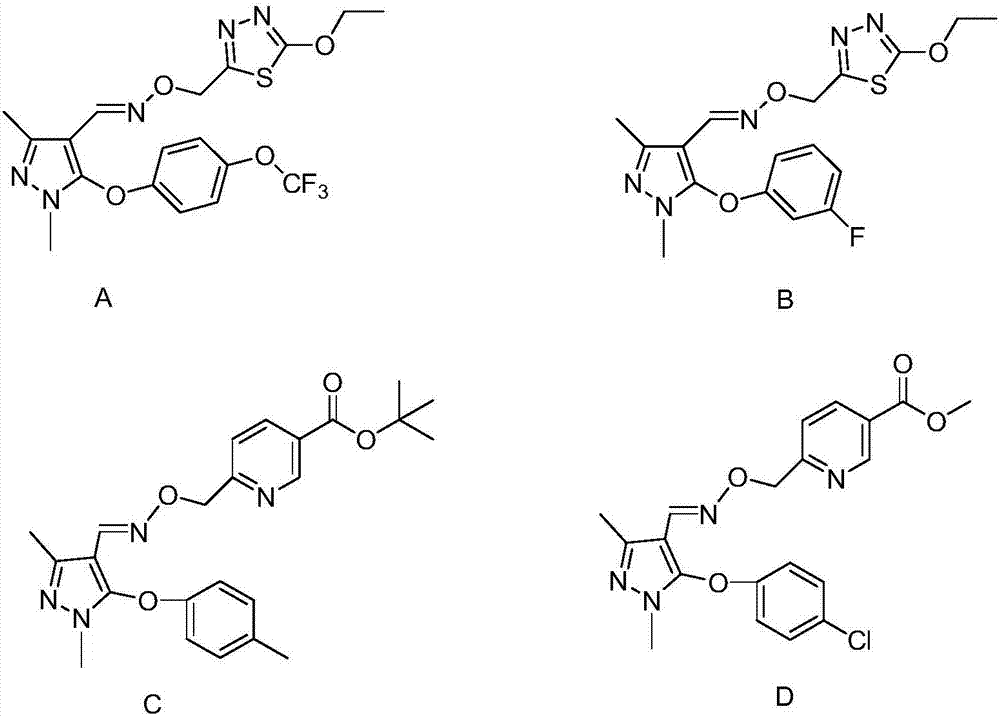
Pyrazole oxime ether compound containing pyrazole biphenyl structure, and preparation method and application thereof
A technology of pyrazole biphenyl and pyrazole oxime ether, which is applied in the fields of organic chemistry and pesticides to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 4mmol of compound IIIa was dissolved in 30mL of acetonitrile, then 10mmol of potassium carbonate was added, and 5mmol of intermediate II was added thereto at room temperature. After the addition was complete, it was heated to reflux for 14 hours. The reaction solution was cooled to room temperature, suction filtered, the mother liquor was concentrated under reduced pressure, and the obtained residue was separated and purified by column chromatography to obtain the target compound Ia; 1 H NMR (400MHz, CDCl 3 ): δ7.91(d, J=2.4Hz, 1H, Pyrazole-H), 7.82(s, 1H, CH=N), 7.72(d, J=2.0Hz, 1H, Pyrazole-H), 7.63(d ,J=8.4Hz,2H,Ar-H),7.38(d,J=8.4Hz,2H,Ar-H),7.16-7.20(m,1H,Ar-H),6.90(d,J=7.6Hz ,1H,Ar-H),6.66-6.70(m,2H,Ar-H),6.46(t,J=2.4Hz,1H,Pyrazole-H),5.03(s,2H,CH 2 ),3.59(s,3H,N-CH 3 ),2.38(s,3H,CH 3 ),2.31(s,3H,CH 3 ).
Embodiment 2
[0047]
[0048] 4 mmol of compound IIIb was dissolved in 20 mL of acetonitrile, and 7 mmol of intermediate II and 8 mmol of cesium carbonate were added thereto at room temperature. After the addition was completed, the reaction was heated to reflux for 13 hours. The reaction was stopped, and the reaction solution was rotary evaporated to dryness under reduced pressure, and the resulting residue was separated and purified by column chromatography to obtain the target compound Ib; 1 H NMR (400MHz, CDCl 3 ): δ7.91(d, J=2.4Hz, 1H, Pyrazole-H), 7.81(s, 1H, CH=N), 7.72(d, J=1.6Hz, 1H, Pyrazole-H), 7.63(d ,J=8.4Hz,2H,Ar-H),7.38(d,J=8.4Hz,2H,Ar-H),7.08(d,J=8.4Hz,2H,Ar-H),6.77(d,J =8.8Hz, 2H, Ar-H), 6.46(t, J=2.0Hz, 1H, Pyrazole-H), 5.03(s, 2H, CH 2 ),3.59(s,3H,N-CH 3 ),2.37(s,3H,CH 3 ),2.30(s,3H,CH 3 ).
Embodiment 3
[0050]
[0051] 5 mmol of compound IIIc was dissolved in 20 mL of DMF, and 5 mmol of intermediate II and 14 mmol of sodium carbonate were added thereto at room temperature. After the addition was complete, the temperature was raised to 90° C. and reacted for 16 hours. The reaction was stopped, and the reaction solution was rotary evaporated to dryness under reduced pressure, and the obtained residue was separated and purified by column chromatography to obtain the target compound Ic; 1 H NMR (400MHz, CDCl 3 ): δ7.91(d, J=2.4Hz, 1H, Pyrazole-H), 7.82(s, 1H, CH=N), 7.72(d, J=1.6Hz, 1H, Pyrazole-H), 7.64(d ,J=8.8Hz,2H,Ar-H),7.35(d,J=8.8Hz,2H,Ar-H),7.16(d,J=8.4Hz,2H,Ar-H),6.89(d,J =9.2Hz, 2H, Ar-H), 6.46(t, J=2.0Hz, 1H, Pyrazole-H), 4.98(s, 2H, CH 2 ),3.61(s,3H,N-CH 3 ),2.36(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com