Preparation method of clobazam
A technology of chlorobazam and malonyl chloride, applied in the field of preparation of chlorobazam, can solve problems such as unfavorable industrialized production, high operational risk and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
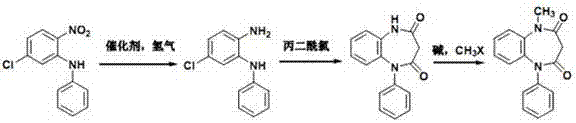
[0023] A preparation method of clobazam, comprising the following steps:
[0024] (1) Synthesis of N-phenyl-5-chloro-1,2-phenylenediamine
[0025] In the high-pressure hydrogenation kettle, the compound 5-chloro-2-nitro- N - Phenylaniline (20 g, 80.43 mmol), Raney-nickel (0.6 g), ethanol (80 mL) and dichloromethane (20 mL). After addition, under 1 bar hydrogen pressure, stir the reaction at room temperature for 1.5 h, stop the reaction, cool and stir in an ice-water bath, a solid precipitates, filter with suction, wash the filter cake with 30 mL of water and 30 mL of petroleum ether successively, and dry in vacuo to obtain a white solid N- Phenyl-5-chloro-1,2-phenylenediamine (15.83 g), mp. 102.5-104.8°C. MS-ESI(m / z): 219.1[M+H] + , 241.1[M+Na] + , 217.0[M-H] - . 1 H-NMR (500MHz, DMSO- d 6 ), δ (ppm): 4.91 (s, 2H, -NH 2 ), 6.70~6.82(m, 5H, Ar-H), 6.95(s, 1H, -NH-), 7.15~7.21(m, 3H, Ar-H),
[0026] (2) 8-Chloro-1-phenyl-1,5-benzodiazepine-2,4(1 H , 3 H )-Diketone Sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com