Paranitrophenetole and preparation method thereof
A technology of nitrophenethyl ether and p-nitrochlorobenzene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as many side reactions, poor product quality, and breakage of ether groups, and achieve The effect of reducing side reactions and reducing the amount of water used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
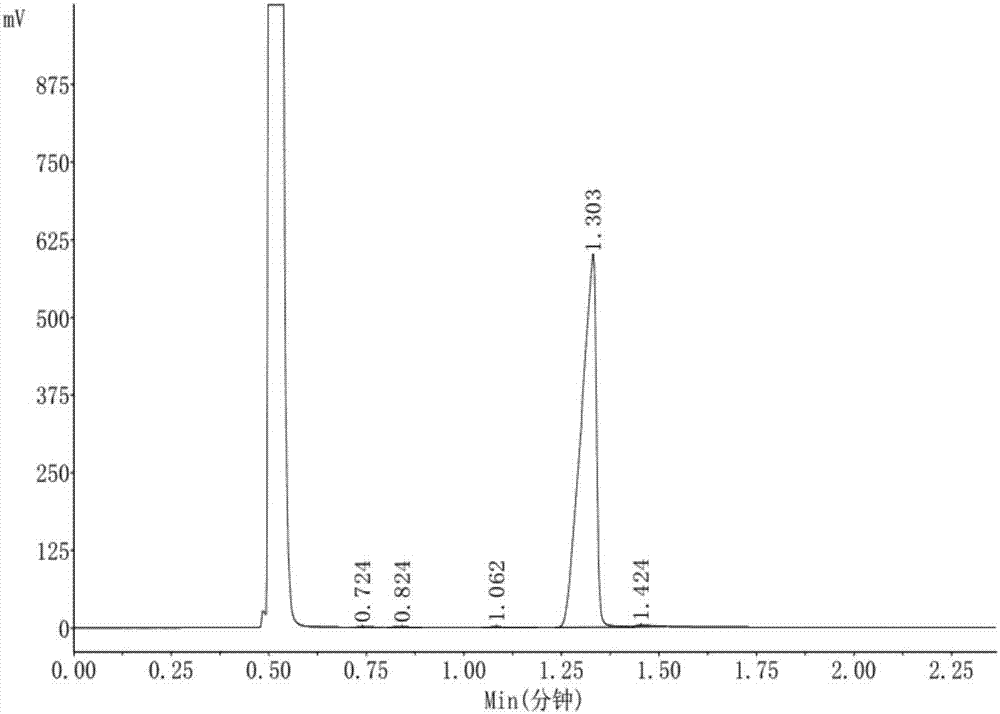
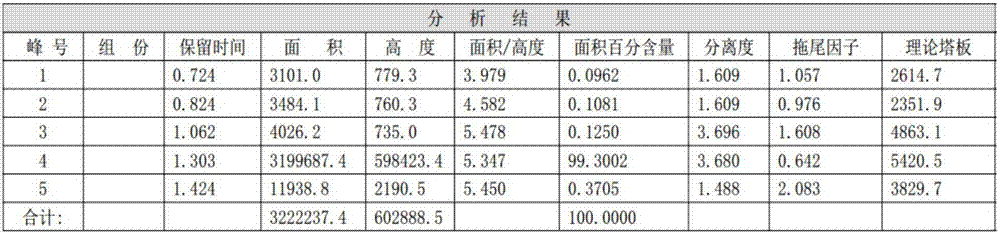
[0012] Put 150g of p-nitrochlorobenzene, 150g of dimethyl sulfoxide, and 65g of ethanol into the reactor, slowly add 39g of solid sodium hydroxide while stirring at a temperature of 50°C, and then stir the reaction, take samples and detect p-nitro The content of chlorobenzene, when the p-nitrochlorobenzene is less than 0.5%, stop the reaction, filter to remove sodium chloride, distill ethanol and water under normal pressure, then distill dimethyl sulfoxide under reduced pressure, and wash the remaining oil layer with hot water , separated the oil layer, cooled and dried to obtain 150 grams of solid product, the content of p-nitrophenetole was 99.30%, and the reaction yield was 94%.
Embodiment 2
[0014] Put 150g of p-nitrochlorobenzene, 150g of dimethyl sulfoxide, and 60g of ethanol into the reactor, slowly add 39g of solid sodium hydroxide while stirring at a temperature of 50°C, and then stir the reaction, take samples and detect p-nitro The content of chlorobenzene, when the p-nitrochlorobenzene is less than 0.5%, stop the reaction, filter to remove sodium chloride, distill ethanol and water under normal pressure, then distill dimethyl sulfoxide under reduced pressure, and wash the remaining oil layer with hot water , separated the oil layer, cooled and dried to obtain 148 grams of solid product, the content of p-nitrophenetole was 99.08%, and the reaction yield was 93%.
Embodiment 3
[0016] Put 150g of p-nitrochlorobenzene, 150g of dimethyl sulfoxide, and 70g of ethanol into the reactor, slowly add 39g of solid sodium hydroxide while stirring at a temperature of 50°C, and then stir the reaction, take samples and detect p-nitro The content of chlorobenzene, when the p-nitrochlorobenzene is less than 0.5%, stop the reaction, filter to remove sodium chloride, distill ethanol and water under normal pressure, then distill dimethyl sulfoxide under reduced pressure, and wash the remaining oil layer with hot water , separated the oil layer, cooled and dried to obtain 150 grams of solid product, the content of p-nitrophenethyl ether was 99.17%, and the reaction yield was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com