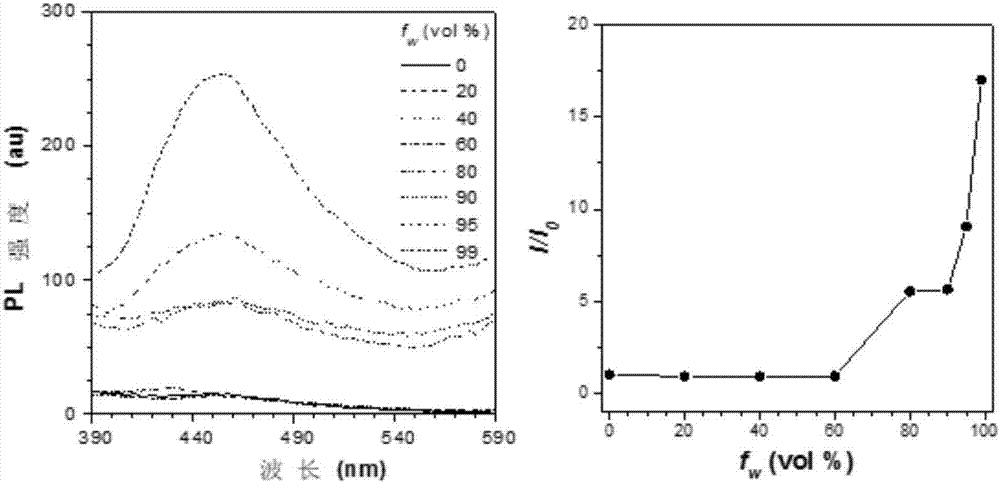
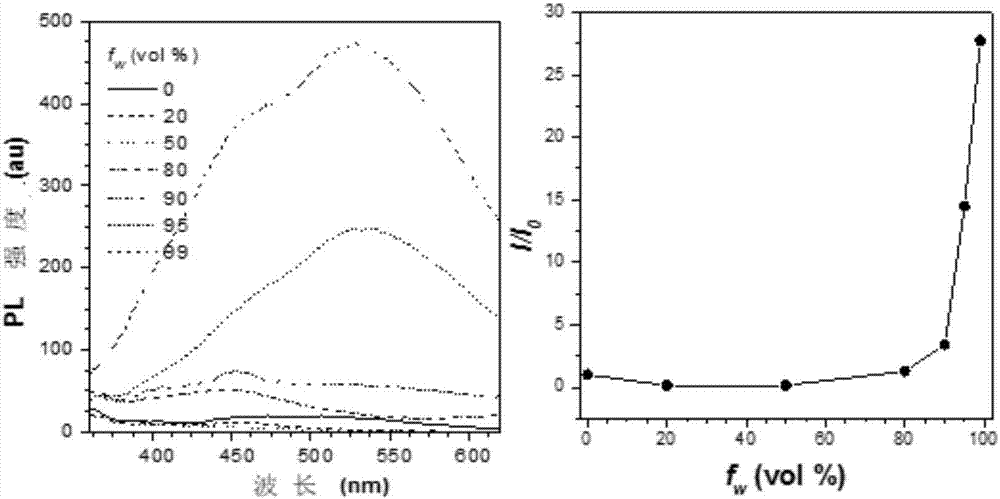
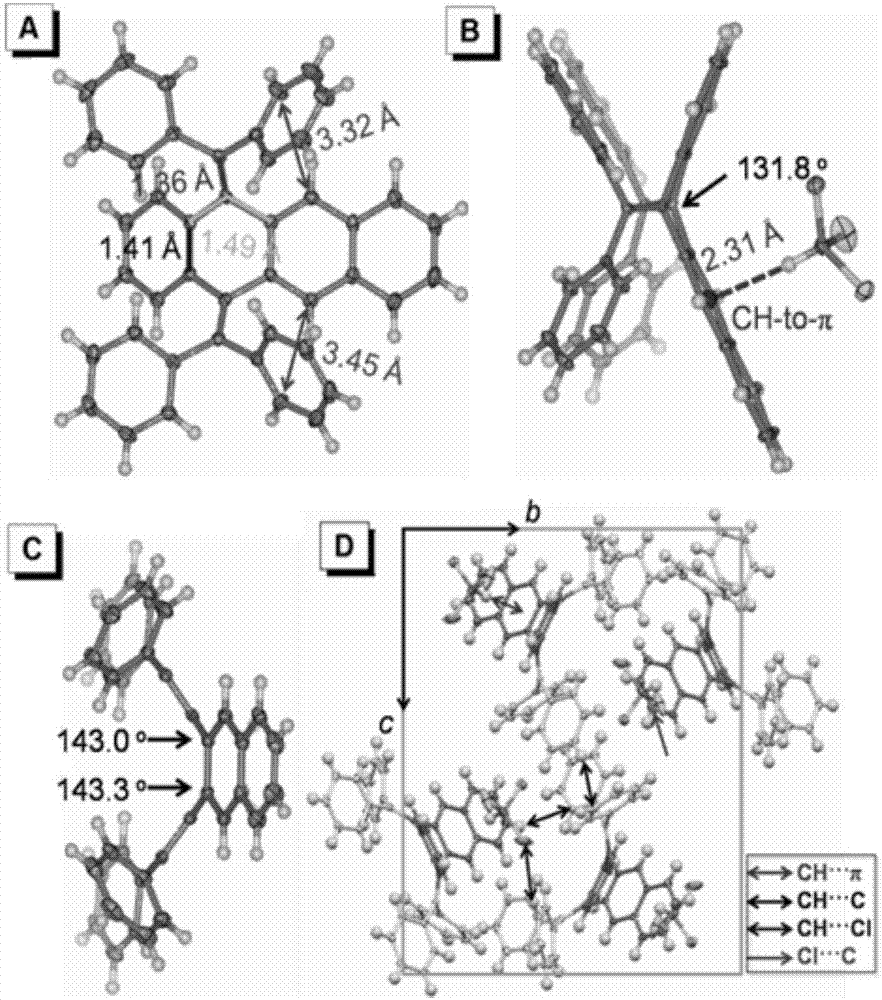
Bi(diaryl methylene)-dihydro acene compound aggregation-induced emission and aggregation-promoting photochromism
A compound, dialkylamino technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as unclear mechanism of RIR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Step (1): Preparation of 5,12-bis(dibromomethylene)-5,12-dihydrotetracene
[0102] CBr 4 (8.6g, 25.9mmol) with PPh 3 (13.6g, 51.8mmol) in 100mL of dry toluene, stirred at room temperature for 30 minutes, then added 5,12-tetrabenzoquinone (2.06g, 8.0mmol) in one portion, and the solution was heated to reflux for 24 hours. The reaction mixture was cooled to room temperature, and the solid was removed by filtration and washed with toluene. The filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was ground by adding ethanol, and the pure product (4.3 g) was obtained after filtration with a yield of 95%.
[0103] 1H-NMR (CDCl 3 )δ (ppm): 8.26 (s, 2H), 7.86 (m, 4H), 7.3 (dd, J 1 =6.4Hz,J 2 =3.2Hz,2H),7.31(dd,J 1 =5.6Hz,J 2 =3.2Hz,2H). 13 C-NMR (CDCl 3 )δ (ppm): 90.59, 127.25, 127.33, 127.44, 127.93, 128.29, 131.79, 133.36, 136.17, 139.73. MALDI-TOF mass: m / z=569.7479 (cacld.569.7475).
[0104] Step (2): Preparation of 5,12-...
Embodiment 2
[0109] To a solution of 5,12-bis(dibromomethylene)-5,12-dihydronaphthacene (2.26 g, 4.0 mmol) and 2-thionylboronic acid (4.10 g, 32 mmol) in 100 mL of toluene, Add 5 mL of ethanol and 5 mL of water. The mixture was stirred at room temperature with nitrogen bubbled through for 30 minutes. Then add K all at once 2 CO 3 (5.52g, 40mmol) and Pd(PPh 3 ) 4 (0.23g, 0.20mmol), and the mixture was heated to reflux for 24 hours. The reaction mixture was cooled to room temperature, filtered through a filter plate (silica gel thickness: 5 cm), washed with dichloromethane until the filtrate was clear. The filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was ground by adding ethanol, and the product (1.86 g) was obtained after filtration with a yield of 80%.
[0110] After the product was dissolved in chloroform, it was placed in a hexane environment, and high-purity colorless crystals were obtained after 2 days.
Embodiment 3
[0112]
[0113] Method S1 Synthesis of 9,10-bis(diarylmethylene)-9,10-dihydroanthracene (R-DHA)
[0114] Reagents and conditions: (i) CBr 4 ,PPh 3 , toluene, 80°C; (ii) arylboronic acid, [PPh 3 ] 4 Pd, K 2 CO 3 , toluene, 110°C.
[0115] Step (1): Synthesis of 9,10-bis(dibromomethylene)-9,10-dihydroanthracene:
[0116] CBr 4 (14.940g, 45.00mmol) and PPh 3 (20.960g, 80mmol) in 100mL of dry toluene was stirred at room temperature for 20 minutes, then anthracene-9,10-dione (2.080g, 10mmol) was added in one portion, and the solution was heated to 80°C for 24 hours. The reaction mixture was cooled to room temperature, and the solid was removed by filtration, washed with toluene, and the filtrate was rotovapped to obtain a crude product. The crude product was ground by adding ethanol, and the pure product (4.120 g) was obtained after filtration with a yield of 78%. 1 H-NMR (CDCl 3 ), δ(ppm): 7.83(m, J=3.2Hz, 4H), 7.28(m, J=3.2Hz, 4H); 13 C-NMR (CDCl 3 )δ (ppm): 139.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com