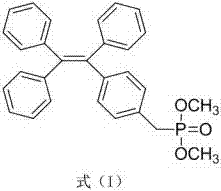
Novel chiral aggregation-induced emission material and preparation method and application thereof
A new type of aggregation-induced luminescence technology, applied in luminescent materials, organic chemical methods, material excitation analysis, etc., can solve problems such as aggregation-induced fluorescence quenching, and achieve great application prospects and good optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Preparation of chiral aggregation-induced luminescence materials
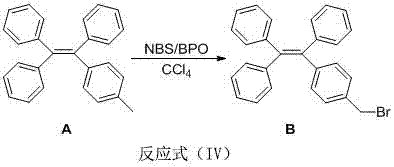
[0059] (1) Synthesis of Compound A: Under the protection of argon, bromotriphenylethylene (800 mg, 2.39 mmol, 1.00 eq), p-tolylboronic acid (486.7 mg, 3.58 mmol, 1.50 eq) and tetrabutyl bromide Ammonium (76.9 mg, 0.24 mmol, 0.10 eq) was placed in a 25 mL inclined two-neck round bottom flask, and toluene (10 mL) and 2 mol / L K 2 CO 3 (4mL). After stirring at 90°C for 10 minutes, tetrakis(triphenylphosphine)palladium (95.4 mg, 0.08 mmol, 0.03 eq) was added and the reaction was continued for 11 hours. After the reaction, extracted three times with ethyl acetate, anhydrous MgSO 4 dry. After filtration, the organic solvent was distilled off under reduced pressure, and the crude product was separated by silica gel column chromatography (petroleum ether) to obtain 825.7 mg of white solid A, with a yield of 99%;
[0060] The synthetic route of the step (1) is as follows:
[0061]
[0062] The compound...
Embodiment 2
[0083] 1. Preparation of chiral aggregation-induced luminescence materials
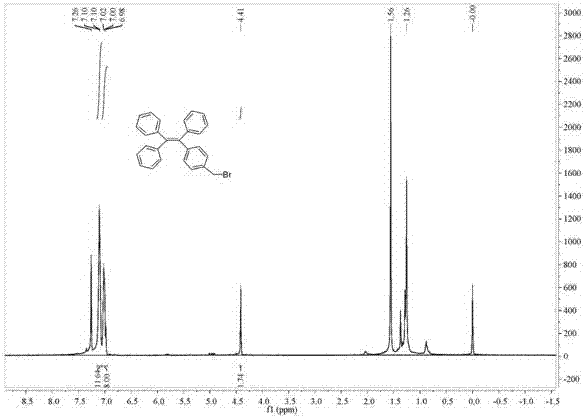
[0084] (1) Synthesis of Compound A: Under nitrogen protection, bromotriphenylethylene (500 mg, 1.49 mmol, 1.00 eq), p-tolylboronic acid (304 mg, 2.24 mmol, 1.5 eq) and tetrabutyl bromide Ammonium (48 mg, 0.15 mmol, 0.10 eq) was placed in a 25 mL inclined two-necked round bottom flask, and toluene (5.0 mL) and 2 mol / L of K 2 CO 3 (2.7 mL). After stirring at 90°C for 10 minutes, tetrakis(triphenylphosphine)palladium (59.6 mg, 0.05 mmol, 0.03 eq) was added and the reaction was continued for 15 hours. After the reaction, extracted three times with ethyl acetate, anhydrous MgSO 4 dry. After filtration, the organic solvent was evaporated under reduced pressure, and the crude product was separated by silica gel column chromatography (petroleum ether) to obtain 392 mg of white solid A with a yield of 76%;
[0085] (2) Synthesis of compound B: under nitrogen protection, compound A (173 mg, 0.5 mmol, 1.00 ...
Embodiment 3
[0089] 1. Preparation of chiral aggregation-induced luminescence materials
[0090] (1) Synthesis of Compound A: Under the protection of argon, bromotriphenylethylene (600 mg, 1.79 mmol, 1.00 eq), p-tolylboronic acid (365 mg, 2.68 mmol, 1.50 eq) and tetrabutyl bromide Ammonium (58 mg, 0.18 mmol, 0.10 eq) was placed in a 25 mL inclined two-necked round bottom flask, and toluene (5 mL) and 2 mol / L K 2 CO 3 (3 mL). After stirring at 90°C for 5 minutes, tetrakis(triphenylphosphine)palladium (72.4 mg, 0.06mmol, 0.035eq) and toluene (1 mL) were added, and the reaction was continued for 8 hours. After the reaction, extracted three times with ethyl acetate, anhydrous MgSO 4 dry. After filtration, the organic solvent was evaporated under reduced pressure, and the crude product was separated by silica gel column chromatography (petroleum ether) to obtain 620 mg of white solid A with a yield of 99%;
[0091] (2) Synthesis of compound B: under the protection of argon, compound A (600...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com