Synthesis of novel organic luminescent material containing carbazolyl toluylene derivant structure and application thereof
A technology of carbazolyl stilbene and light-emitting materials, which is applied in the fields of light-emitting materials, organic chemistry, and material excitation analysis. It can solve the problems of poor OLED stability, low luminous efficiency and intensity, and short life, and achieve high glass transition. Effects of temperature, ease of purification, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
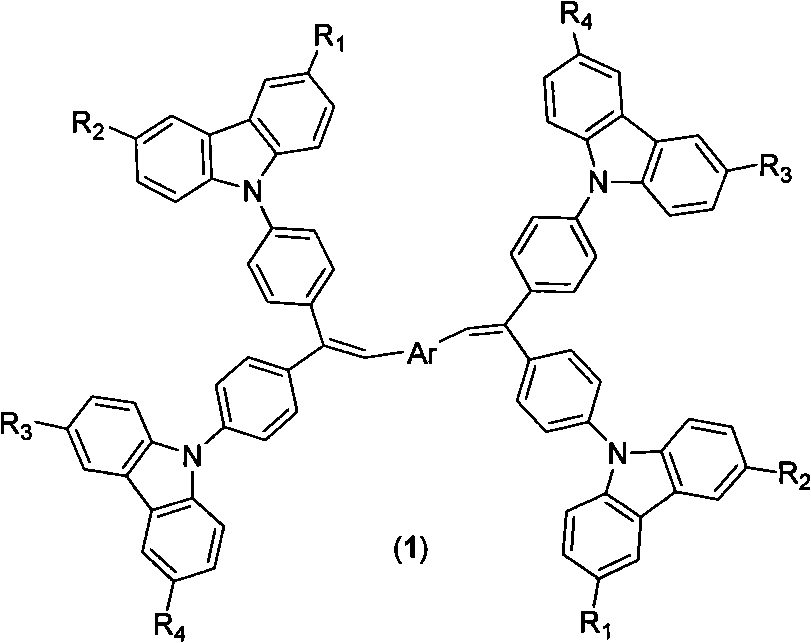
[0016] Synthesis of 4,4'-bis(2,2-bis(4-(9H-carbazolyl)phenyl)vinyl)biphenyl:
[0017] (1) Synthetic intermediate bis(4-(9H-carbazolyl)phenyl)methanone
[0018] Dissolve 25g (0.15mol) of carbazole in 200mL of DMF, add 20g (0.19mol) of potassium tert-butoxide under stirring and raise the temperature to 60°C for 30min, then add 14.8g (0.068mol) of difluorobenzophenone, and then raise the temperature to 110 ℃ reaction 12h. Stop the reaction, pour into 500mL water for precipitation after cooling, filter with suction, and wash with water several times. Dissolve the solid in 150mL of dichloromethane to form a solution, add an appropriate amount of anhydrous sodium sulfate to dry, and filter. Add 150mL of acetone to the filtrate, dichloromethane and most of the acetone are evaporated by a rotary evaporator to obtain a precipitate, filtered by suction, washed 3 times with a small amount of acetone, and vacuum-dried to obtain 32g of light yellow powder with a yield of 92%.
[0019] ...
Embodiment 2
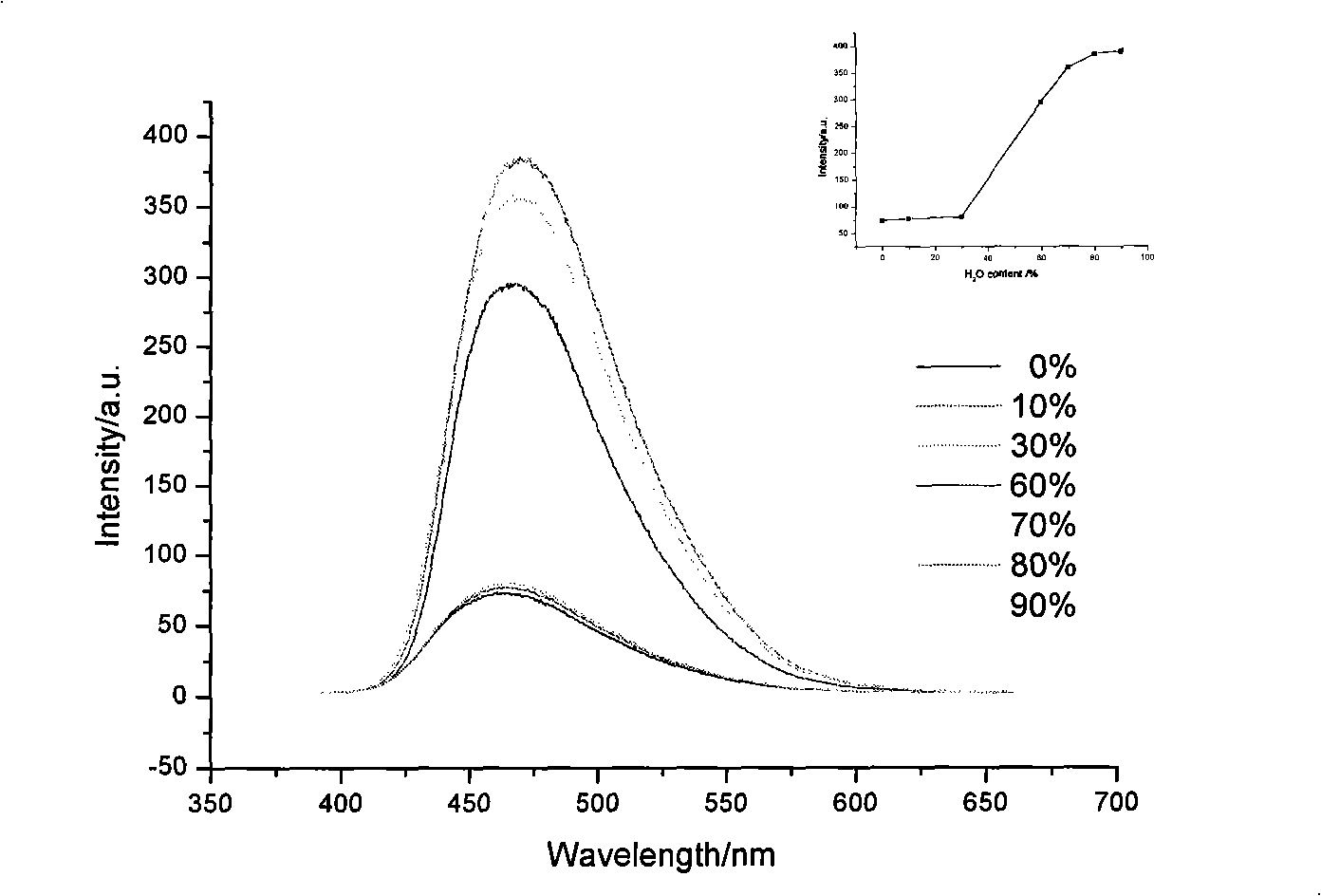
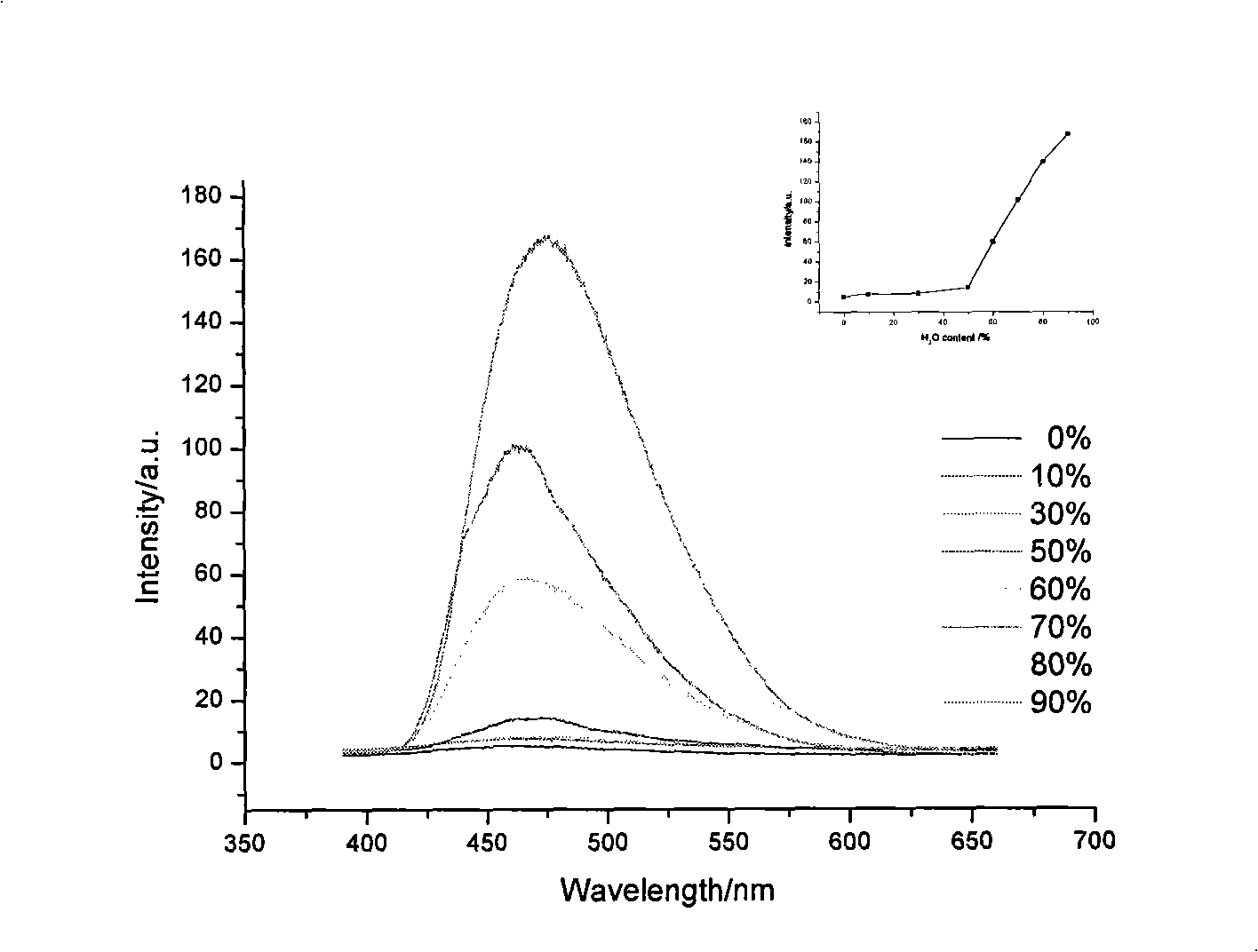
[0024] The synthetic method of 2,5-bis(4-(2,2-bis(4-(9H-carbazolyl)phenyl)vinyl)phenyl)-1,3,4-oxadiazole) refers to the example 1, the phosphonate ylide reagent used is tetraethyl (4,4'-(1,3,4-oxadiazole-2,5-diyl) bis(4,1-phenylene)) bis(sub- Methyl) bisphosphonate. The yield was 65%. lambda max em 471nm, Φ FL is 2.5%, Tg is 192°C, and Td is 495°C.
[0025]
Embodiment 3
[0027] Synthesis of 4,4'-bis(2,2-bis(4-(3,6-di-tert-butyl-9H-carbazolyl)phenyl)vinyl)biphenyl:
[0028] (1) Synthesis of intermediate bis(4-(3,6-di-tert-butyl-9H-carbazolyl)phenyl)methanone
[0029] 4.2 g (0.015 mol) of 3,6-di-tert-butyl-9H-carbazole was dissolved in 50 mL of DMF, 2 g (0.019 mol) of potassium tert-butoxide was added under stirring and the temperature was raised to 60 ° C for 30 min, then difluoro di 1.5 g (0.0068 mol) of benzophenone was heated to 110° C. for 12 hours. Stop the reaction, pour it into 500mL water after cooling to produce a precipitate, filter it with suction, and wash it with water several times. Dissolve the solid in 150mL of dichloromethane to form a solution, add an appropriate amount of anhydrous sodium sulfate to dry, and filter. Add 150mL of acetone to the filtrate, dichloromethane and most of the acetone are evaporated by a rotary evaporator to obtain a precipitate, filtered by suction, washed 3 times with a small amount of acetone, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com