Use of FGFR mutant gene panels in identifying cancer patients that will be responsive to treatment with an FGFR inhibitor
A technology for FGFR3G370C, FGFR3, applied in the field of using FGFR mutant genome to identify cancer patients who will respond to treatment with FGFR inhibitors, can solve problems such as patients without FGFR changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1 - Plasmid DNA isolation and purification
[0200] The following is an exemplary procedure for preparing FGFR fusion plasmid DNA.
[0201] Equipment needed: centrifuge, capable of 1500 x g; microcentrifuge; pipette, positive displacement or exhaust; vortexer; nanodtop spectrophotometer; 37 °C shaker / incubator; and set at 37 ℃ oven.
[0202] Materials needed: frozen glycerol bacterial stock containing plasmid DNA; Kanamycin LB Agar Plates (Teknova #L1155); LB Broth (Life Technologies #10855-021); Kanamycin (Sigma #K0254); Plasmid Purification Kit (Qiagen # 12123); Pure Ethanol (Sigma Aldrich # E7023); Isopropanol (Sigma Aldrich #W292907); Nuclease-free water (without DEPC treatment) (from IDT or Ambion # AM9932); RNase barrier (filter) tips; RNase-free microtubes (1.5 to 2 mL VWR# 10011-724); serological pipettes; and 14ml round bottom tubes (VWR#352057).
[0203] To recover bacteria from the glycerol stock, use the tip of a sterile pipette to scrape frozen...
Embodiment 2
[0205] Example 2 - Generation of NRK cell lines
[0206] Expression vectors expressing each FGFR fusion were constructed. The expression vector was then transfected into normal rat kidney epithelial (NRK) cells. After transfection, stable cell lines were selected in media containing kanamycin. These cells were then grown, mRNA isolated and subjected to a FGFR fusion assay to confirm the presence of specific FGFR fusion mRNA.
Embodiment 3-F
[0207] Example 3-FGFR fusion cell line maintenance
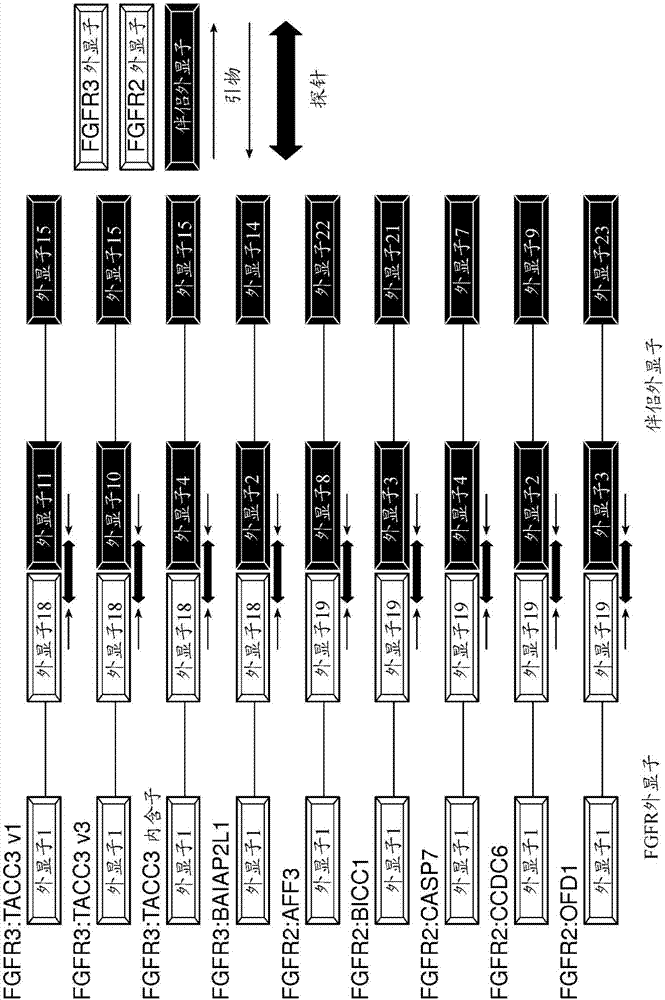
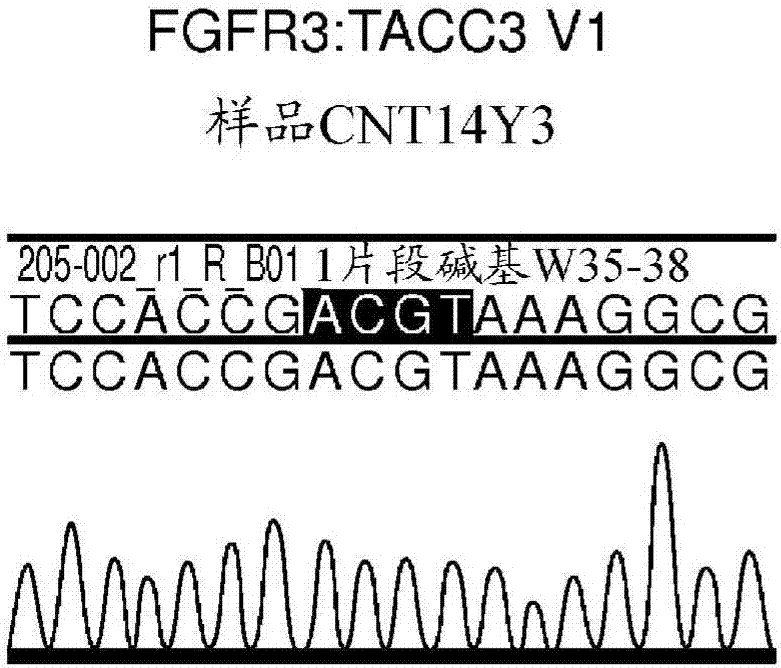
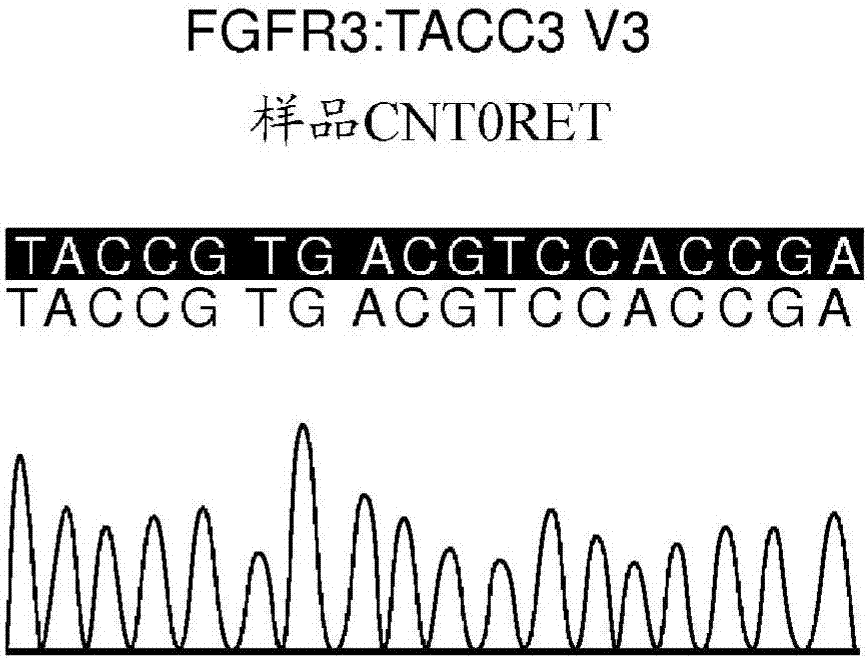
[0208] The following protocol describes exemplary methods for culturing and maintaining NRK FGFR fusion overexpressing cell lines. Cell lines include, but are not limited to: NRK / FGFR3:TACC3v1, NRK / FGFR3:TACC3 v3, NRK / FGFR3:BAIAP2L1, NRK / FGFR2:BICC1, NRK / FGFR2:CASP7, NRK / FGFR2:CCDC6, NRK / FGFR2:AFF3, NRK / FGFR2:OFD1 and NRK / EMPTY VECTOR (plasmid control).
[0209] Required equipment: Biological safety cabinet, equipped with a vacuum suction system; CO 2 Incubator, set to 5% CO 2 and 37°C; a -80°C refrigerator; a liquid nitrogen tank; a water bath, set at 37°C; and a microscope.
[0210] Materials needed: Serological pipettes; Tissue culture flasks (T75 VWR # BD353136 and / or T150 VWR #15705-074); Tissue culture 0.2 μm filter units (Thermo Scientific #566-0020); Gell's Medium) Cell Culture Medium (Life Technologies, #11965-084); Fetal Bovine Serum (FBS), Certified Heat Inactivated (Life Technologies, #10082147); PenStrep A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com