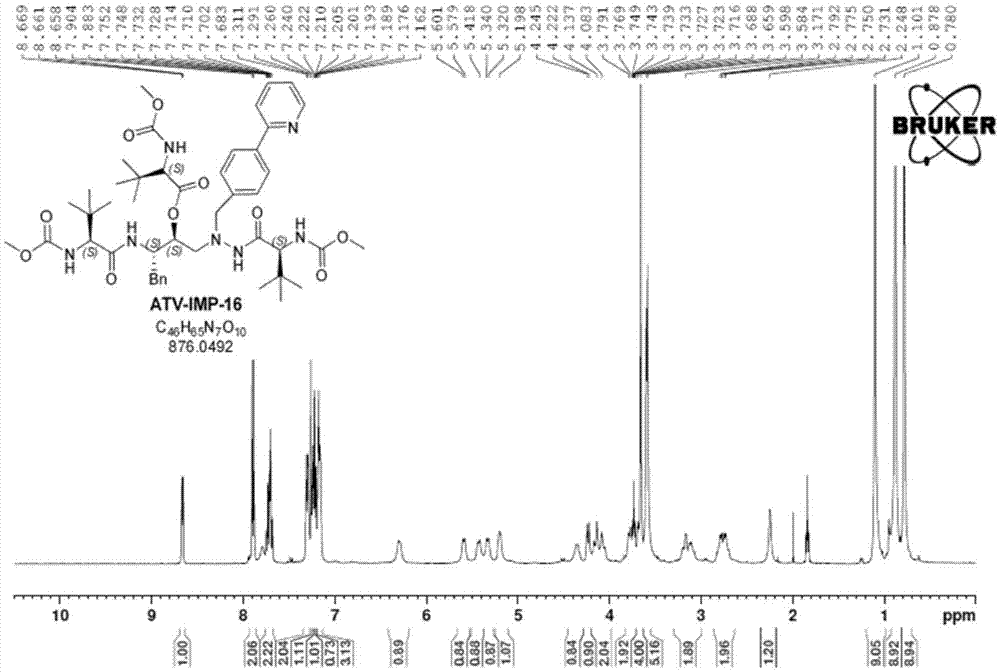
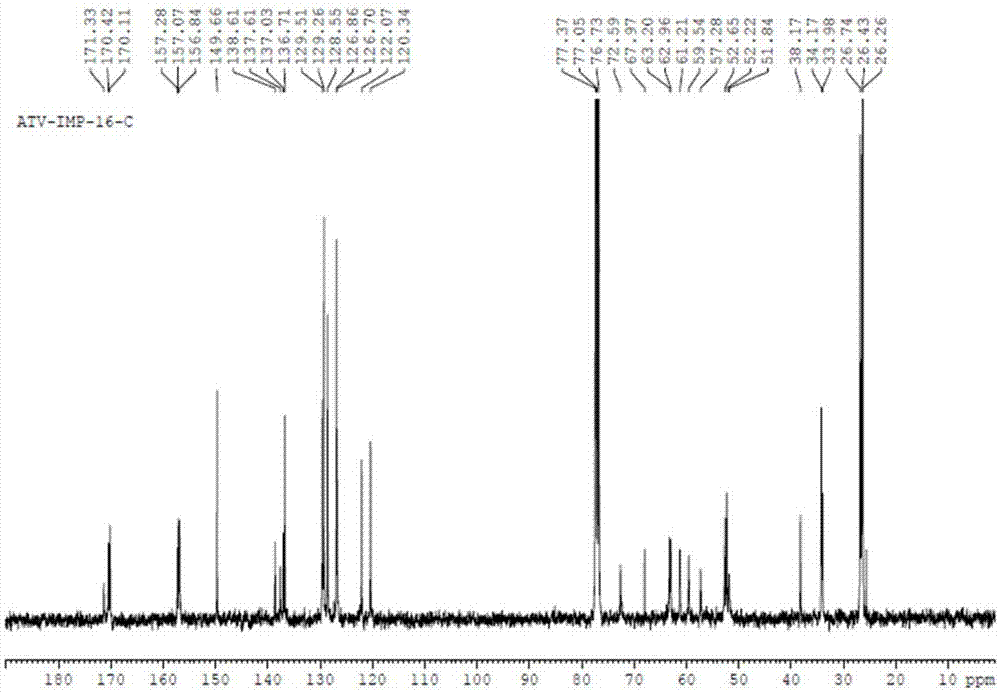
Atazanavir bulk drug impurity or salt thereof, preparation method and applications thereof
A technology of atazanavir and raw material medicine, applied in the field of atazanavir raw material medicine impurity or its salt, its preparation and application, can solve the problems of containing impurities, unable to effectively identify, unable to effectively control the quality of atazanavir, etc. , to achieve the effect of high yield and purity, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Compound N-methoxycarbonyl tert-leucine (3.5g, 18.5mmol), EDCI (3.9g, 21.1mmol), HOBt (2.85g, 21.1mmol) and dichloromethane (40mL) were added into a 250mL three-necked flask at room temperature to respond in the bottle. Stir at 20°C-30°C for 0.5-1 hour, add K 2 HPO 4 Aqueous solution (8.8 g, 5 mL / g), stirring was continued for 1 hour.
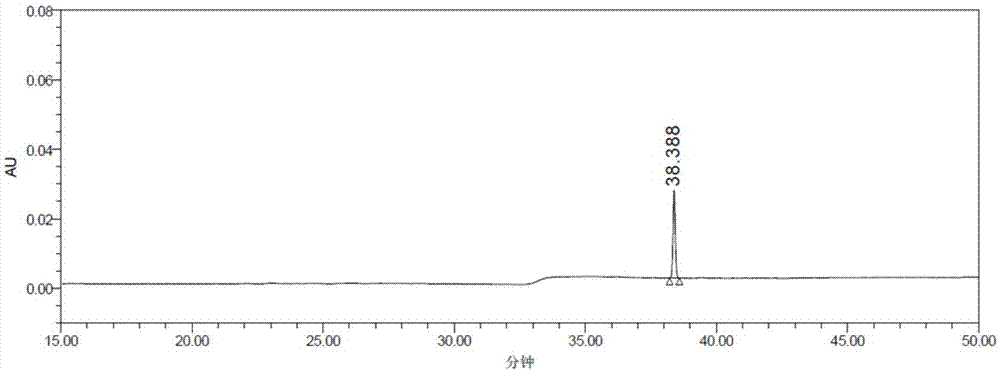
[0037] Dissolve 2.5g (5.3mmol) of the trihydrochloride salt of the compound represented by formula (II) in 15-25ml of water, slowly drop into the above reaction solution, and stir at room temperature for 24 hours; water (100mL×2), NaHCO 3 (100mL×2) and brine (100mL) wash the reaction solution, collect the organic phase, concentrate, and dry to obtain a yellow solid, which is separated by preparative liquid phase (the HPLC figure is shown in image 3 ), collecting the eluent with a retention time of 35 minutes-40 minutes, concentrated and dried to obtain the target compound with a yield of 85%; HPLC purity greater than 98%.
[0038] A...
Embodiment 2
[0055] Compound N-methoxycarbonyl tert-leucine (3.5g, 18.5mmol), EDCI (3.9g, 21.1mmol), HOBt (2.85g, 21.1mmol) and dichloromethane (40mL) were added into a 250mL three-necked flask at room temperature. in the bottle. Stir at 20-30°C for 0.5-1 hour, add K 2 HPO 4 Aqueous solution (8.8 g, 5 mL / g), stirring was continued for 1 hour. Add 2.0 g (5.3 mmol) of the compound shown in formula (II), stir at room temperature for 24 hours, water (100 mL×2), NaHCO 3 (100mL×2) and brine (100mL) to wash the reaction solution, collect the organic phase, concentrate, and dry to obtain a yellow solid, which is separated by liquid phase preparation, and the eluate with a retention time of 35 minutes to 40 minutes is collected, concentrated and dried to obtain The yield of the target compound (the structure identification data is the same as that in Example 1) is 90%, and the HPLC purity is greater than 98%.
[0056] Other experimental conditions and results are as follows:
[0057]
Embodiment 3
[0059] Compound N-methoxycarbonyl tert-leucine (3.5g, 18.5mmol), EDCI (3.9g, 21.1mmol), HOBt (2.85g, 21.1mmol) and dichloromethane (40mL) were added into a 250mL three-necked flask at room temperature. in the bottle. Stir at 20-30°C for 0.5-1 hour, add K 2 HPO 4 Aqueous solution (8.8 g, 5 mL / g), stirring was continued for 1 hour.
[0060] Dissolve 3.8g (8.0mmol) of the compound of formula (II) in 15-25ml of water, slowly drop it into the above reaction solution, and stir at room temperature for 24 hours; water (100mL×2), NaHCO 3 (100mL×2) and brine (100mL) wash the reaction solution, collect the organic phase, concentrate, and dry to obtain a yellow solid, which is separated from the preparation liquid phase (the specific conditions are the same as in Example 1, and its HPLC figure is shown in Figure 4 ), collecting and washing the eluent with a retention time of 20 minutes to 30 minutes, concentrating and drying to obtain atazanavir, the HPLC purity is greater than 98%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com