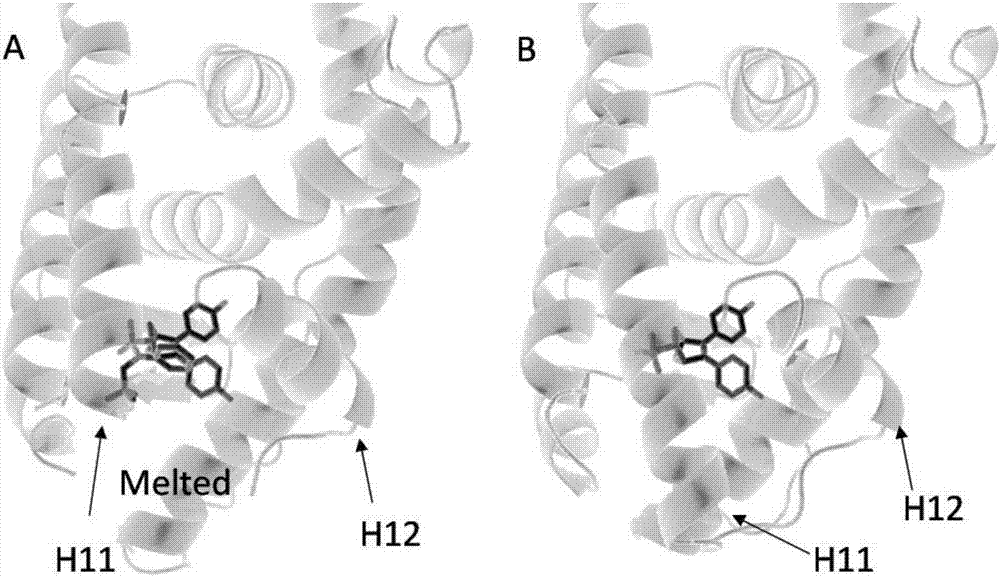
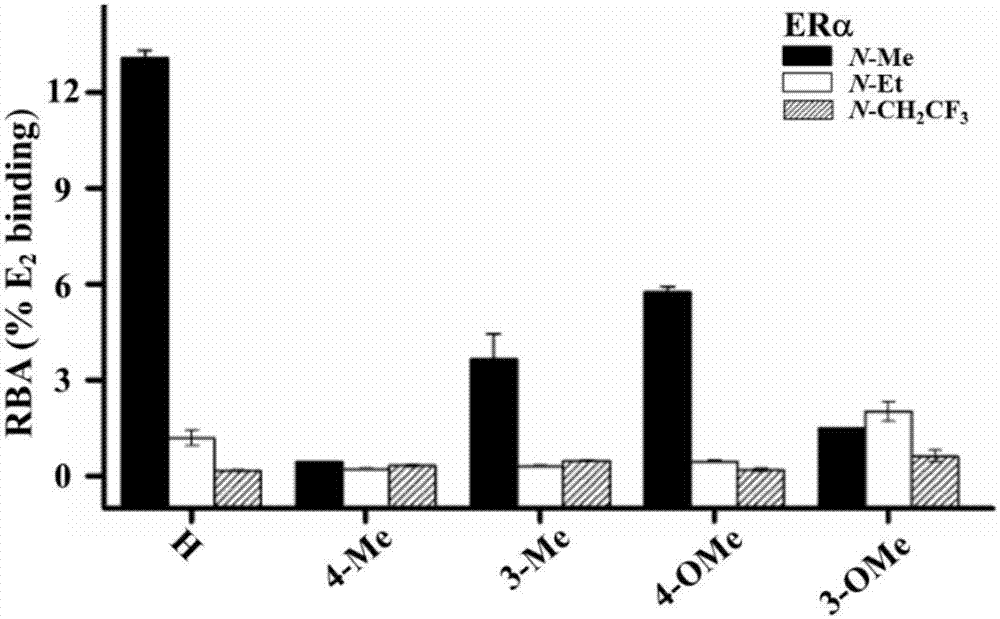
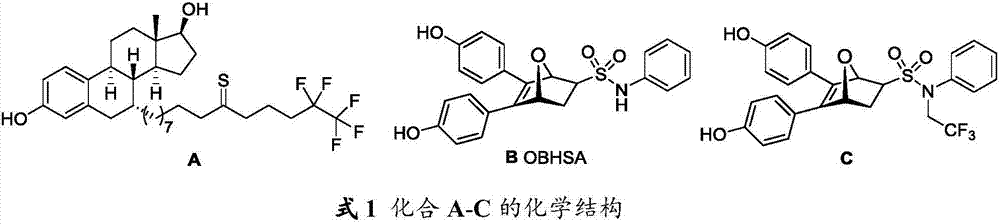
Oxygen bridge bis-heptylene sulfonamide compound containing suberic acid monoanilide group as well as synthesizing method, application and anti-breast cancer drug composition of oxygen bridge bis-heptylene sulfonamide compound
A technology of suberoyl anilide and bisheptene sulfonamide, applied in the field of medicine, can solve the problems of ERβ without any activation activity and low endogenous value-added activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: 5,6-bis(4-hydroxyl)-7-oxobicyclo[2.2.1]-5-heptene-2-sulfonyl-(4-anilinoformylheptanoic acid methyl ester)-amine Preparation of (32a)
[0123]
Embodiment 2
[0124] Weigh 3,4-bis(4-hydroxy-phenyl)furan (200mg, 0.793mmol) and ethylene 4-(4-(7-anicarboylheptanoic acid methyl ester))-sulfonamide (350.7mg, 0.952 mmol) was placed in a two-necked round-bottom bottle of 50mL and then slowly warmed up to 90° C., spin-dried after 3 hours of reaction, and separated and purified by direct column chromatography. The eluent ratio was dichloromethane:methanol=60:1 to obtain 413.6 mg white solid, yield 94%, m.p.108-110°C; 1 H NMR (400MHz, Acetone-d 6 )δ 9.29(s,1H,-CONH-),8.87(s,1H),7.76(s,1H),7.50(d,J=8.4Hz,1H),7.22(t,J=8.0Hz,1H) ,7.16(d,J=8.4Hz,2H),7.10(d,J=8.8Hz,1H),7.02(d,J=8.8Hz,2H),6.78(d,J=8.4Hz,2H),6.74 (d,J=8.4Hz,2H),5.52(s,1H),5.33(d,J=4.0Hz,1H),3.60(s,3H),3.55(m,1H),2.40(t,J= 7.2Hz, 2H), 2.34(m, 1H), 2.28(t, J=7.6Hz, 2H), 1.98(m, 1H), 1.69(t, J=7.2Hz, 2H), 1.57(t, J= 6.4Hz,2H),1.35(m,4H). 13 C NMR (100MHz, Acetone-d 6)δ174.32,172.65, 158.24,158.04,141.95,141.33,139.74,138.27,130.47,129.79,129.37,125.04,124.56, 116.47,116.41,116.03...
Embodiment 3
[0127] Example 3: 5,6-bis(4-hydroxyl)-7-oxobicyclo[2.2.1]-5-heptene-2-N-ethylsulfonyl-(7-anilinoformylheptanoic acid methyl Preparation of ester)-amine (33b)
[0128]
[0129] The preparation method is as in Example 1, the product is light yellow powder, the yield is 95%, m.p.105-108°C; 1 H NMR (400MHz, Acetone-d 6 )δ9.26(s,1H,-CONH-),8.70(s,2H,-OH),7.75(s,1H),7.64(d,J=8.4Hz,1H), 7.25(t,J=8.4 Hz,1H),7.19(d,J=8.0Hz,2H),7.18(d,J=8.0Hz,2H),7.07(d,J=8.0Hz,1H),6.82(d,J=8.4Hz, 2H), 6.80(d, J=8.0Hz, 2H), 5.49(s, 1H), 5.33(d, J=3.6Hz, 1H), 3.82(m, 2H), 3.60(s, 3H), 3.54( m,1H),2.38(t,J=7.6Hz,2H),2.28(t,J=7.2Hz,2H),2.19(m,1H),2.06(m,1H),1.69(t,J=6.4 Hz,2H),1.58(t,J=7.2Hz,2H),1.36(m,4H),1.04(t,J=7.2Hz,3H). 13 C NMR (100MHz, Acetone-d 6 )δ174.28,172.48,158.23,141.95,141.01,140.67, 138.40,130.42,129.99,129.81,129.56,125.26,124.63,124.43,121.00,119.20,116.53,116.39, 85.27,83.70,62.75,51.59,47.07,37.65,34.30 ,31.30,29.61,29.53,26.01,25.52,14.95; HRMS(ESI)calcd for C 35 h 39 N 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com