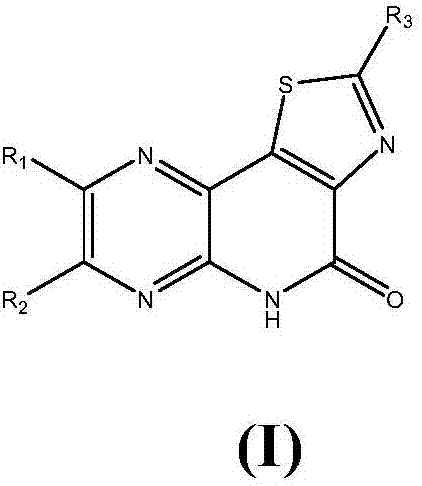
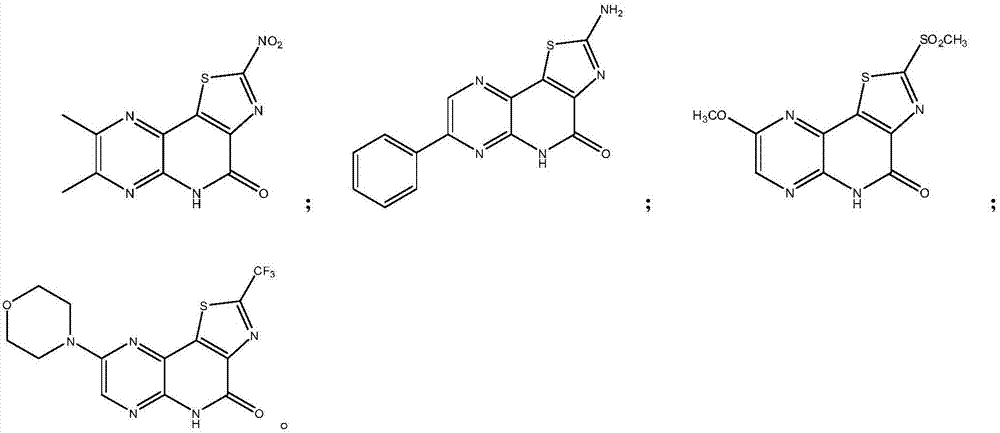
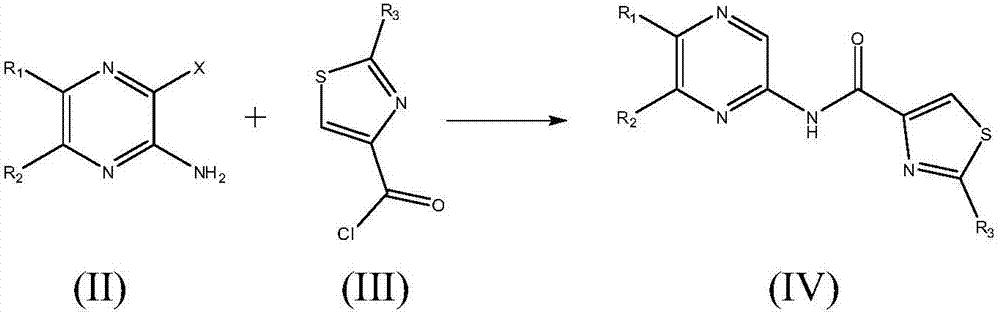
Medicine for preventing and treating myocardial ischemia and preparation method thereof
A pharmacy and compound technology, applied in the field of medicine, can solve problems such as abnormal heart failure, atrioventricular block, and clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] Potassium tert-butoxide (5.60 g, 50 mmol) was added to a solution of 5,6-dimethylpyrazin-2-amine (1.23 g, 10 mmol) in 250 mL of anhydrous THF at room temperature under nitrogen. After stirring for 15 minutes, a solution of 2-nitrothiazole-4-oyl chloride (2.10 g, 11 mmol) in 50 mL of anhydrous THF was added dropwise. The reaction mixture was stirred at room temperature for 4 h, then poured into saturated NaHCO 3 aqueous solution and extracted with EtOAc. The organic phase was washed with saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4 Dried and concentrated to dryness. Purification by flash chromatography on silica gel (cyclohexane / EtOAc: 90 / 10 to 50 / 50) gave N-(5,6-dimethylpyrazin-2-yl)-2-nitrothiazole as a white solid -4-amide 1.69 g (yield: 61.0%).
[0058] The catalyst tetrakis(triphenylphosphine)palladium (0.63mg, 0.55mmol) and potassium acetate (0.98g, 10mmol) were added to N-(5,6-dimethylpyrazine in a microwave reactor) under ni...
Embodiment 2
[0064]
[0065] Potassium tert-butoxide (5.60 g, 50 mmol) was added to a solution of 6-phenylpyrazin-2-amine (1.71 g, 10 mmol) in 250 mL of anhydrous THF at room temperature under nitrogen. After stirring for 15 minutes, a solution of 2-aminothiazole-4-oyl chloride (1.77 g, 11 mmol) in 40 mL of anhydrous THF was added dropwise. The reaction mixture was stirred at room temperature for 5 h, then poured into saturated NaHCO 3 aqueous solution and extracted with EtOAc. The organic phase was washed with saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4 Dried and concentrated to dryness. After purification by flash chromatography on silica gel (n-Hexane / EtOAc: 95 / 5 to 40 / 60), N-(6-phenylpyrazin-2-yl)-2-aminothiazole-4-amide 1.74 was obtained as a gray solid. g (yield: 58.9%).
[0066] The catalyst tetrakis(triphenylphosphine)palladium (0.63mg, 0.55mmol) and potassium acetate (0.98g, 10mmol) were added to N-(6-phenylpyrazine-2- In a solution of (1.48 g, 5 mm...
Embodiment 3
[0073]
[0074] ESI-MS: 313.00[M+H] +
[0075] Elemental analysis: theoretical value / measured value, C(38.46 / 38.51), H(2.58 / 2.55), N(17.94 / 17.88), O(20.49 / 20.59), S(20.53 / 20.47)
[0076] 1 HNMR (400MHz, CDCl 3 )δ: 8.05(s,1H), 7.63(s,1H), 4.02(s,3H), 3.47(s,3H).
[0077] 13 C NMR (400MHz, CDCl 3 )δ: 157.2, 154.1, 153.4, 151.0, 141.4, 139.3, 135.7, 133.6, 133.0, 54.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com