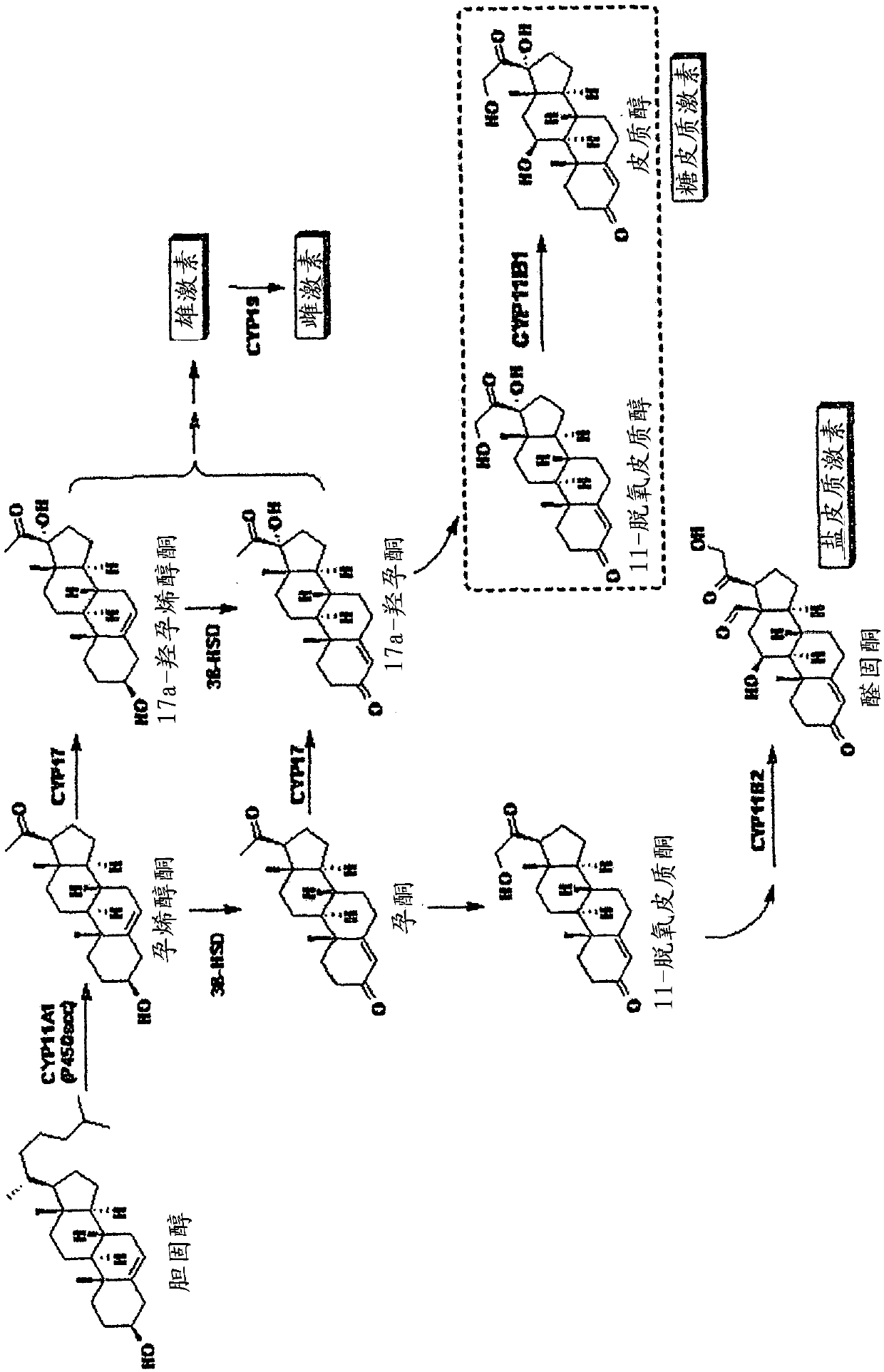
A plant extract and compounds for use in wound healing
A technology of plant extracts and compounds, applied in the direction of plant raw materials, plant/algae/fungus/moss components, drug combinations, etc., can solve the problem of inability to promote skin wound healing in rats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] 1.1. Preparation of extract solution
[0111] Applicants dissolved ~10 mg of Salvia m. Bunge extract (as disclosed in WO2009050451 ) in the required volume of 100% ethanol or 100% DMSO to obtain a 1% (w / v) extract solution. They tested 5 μL of this solution (1% final ethanol or DMSO concentration) in a 500 μL assay incubation volume. From this 1% solution of Danshen extract, they also prepared 1:10 and 1:100 dilutions in 100% ethanol or 100% DMSO. From these solutions, they tested 5 μL in a 500 μL assay incubation volume.
[0112] 1.2. Determination of CYP11B1
[0113] The V79MZh11B1 cell line expressing recombinant human CYP11B1 was supplemented with 5% fetal calf serum (FCS; Sigma), penicillin G (100 U / ml), streptomycin (100 μg / ml), glutamine (2 mM) and sodium pyruvate (1mM) Dulbecco modified Eagle (DME, Sigma)) at 37°C, 5% CO 2 Cultivate in the air. Cells were placed on a 24-well cell culture plate (8 × 10 per well 5 cells) and cultured to confluency in 1 ml D...
Embodiment 2
[0127] MTT cell viability assay
[0128] Place V79MZh11B1 cells on 1ml DME medium in a 24-well cell culture plate (8×10 per well 5 cells) were grown to confluence. On the day of the test, the DME medium was removed, and 450 μl of fresh DME medium containing 5% FCS (containing 5 μl of Danshen extract solution in 100% ethanol) was added to each well. Ethanol (1%) and Triton X-100 (0.0006%) were used as vehicle and positive control, respectively (all final concentrations). All measurements were performed in quadruplicate. At 37°C, 5% CO 2 After incubation for 60 min, 50 μl of fresh DME medium (+5% FCS) was added to each well. After 25 min, the medium was replaced with 500 μl of fresh DME medium (+5% FCS), and 25 μl of MTT solution (5 mg / ml PBS, pH 7.2) was added thereto immediately. After 30 min, all medium was removed and cells were lysed in 250 μl of 0.5% acetic acid (v / v), 10% SDS (w / v) in DMSO. Measuring formazan by spectrophotometry at a wavelength of 570 nm (formaza...
Embodiment 3
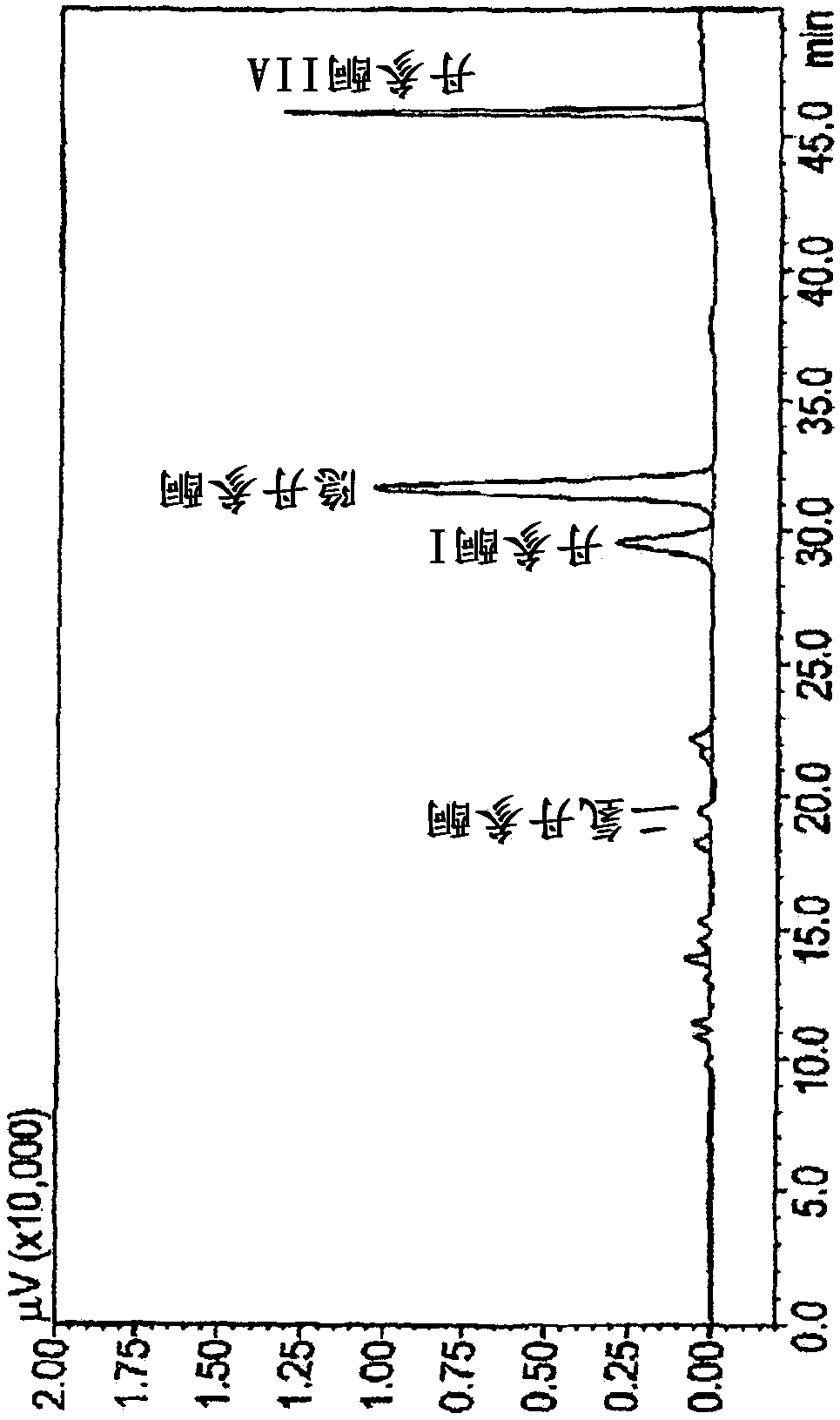
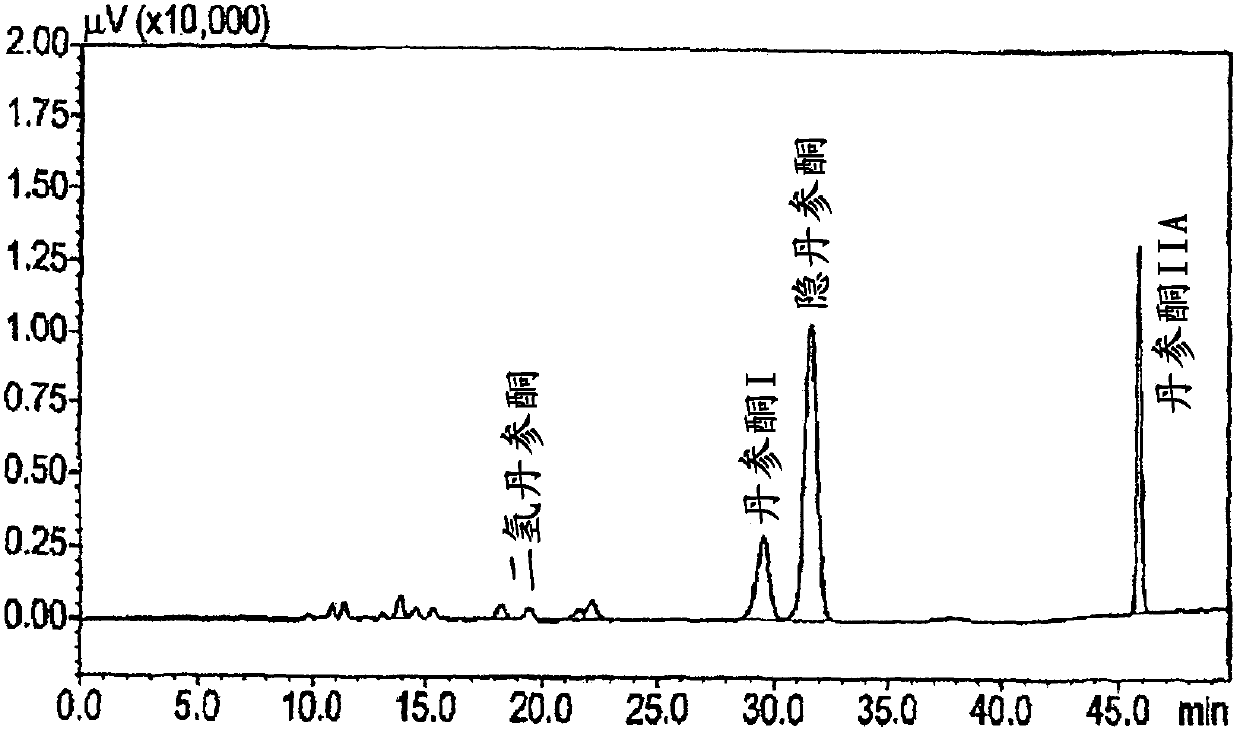
[0136] Considering the activity of the extracts, applicants used the methodology described in Example 1 to observe the activity of some of the tanshinones.
[0137] The tanshinones tested in V79MZh11B1 cells were:
[0138] ●Tanshinone IIA;
[0139] ●Tanshinone I;
[0140] ● Dihydrotanshinone I; and
[0141] ● Cryptotanshinone.
[0142] The results are shown in Table 3 below:
[0143] table 3.
[0144]
[0145] table 3. CYP11B1 inhibition by tanshinone IIA, tanshinone I, dihydrotanshinone I, and cryptotanshinone. Results are mean ± SD of 2 independent experiments.
[0146] As apparent from the results, each tanshinone showed inhibitory activity, the two most potent being dihydrotanshinone (94% inhibition at 10 μM) and tanshinone I (64% inhibition at 10 μM).
[0147] This in itself was unexpected as these two compounds were present in lower amounts in the extract disclosed in WO2009050451 (3.65% and 3.82%, respectively) than cryptotanshinone and tanshinone IIa (18.95...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com