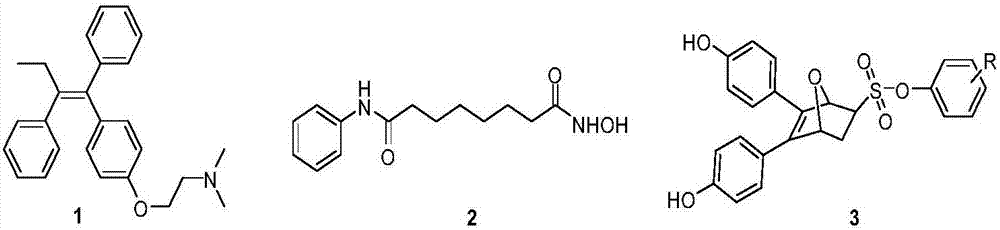
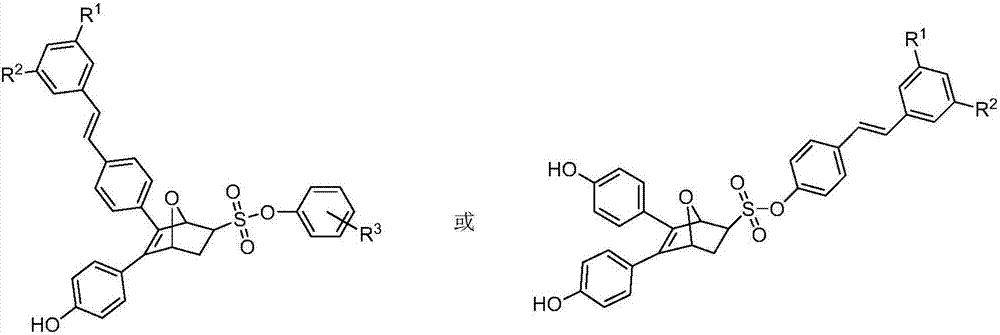
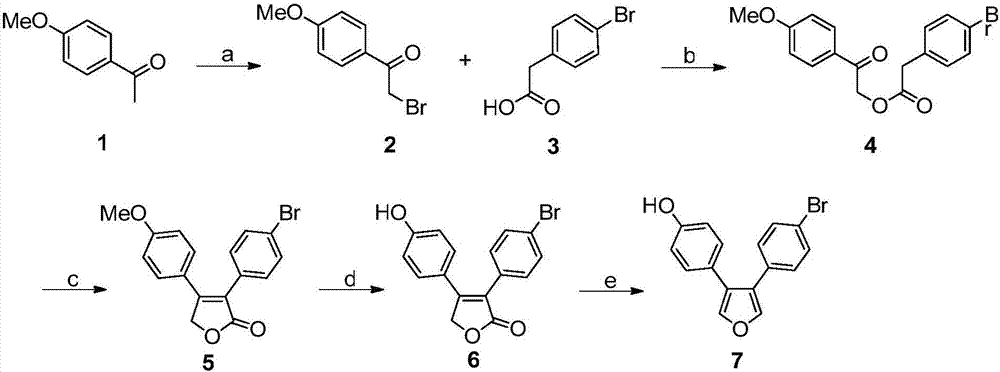
Oxygen bridge dicycloheptene compound containing resveratrol group and its preparation method and use method
A technology of resveratrol and bicycloheptene, applied in the field of medicine, can solve the problems of increasing the risk of endometrial cancer and developing drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: 5-(4-hydroxyphenyl)-6-(4-((E)-3,5-dimethoxystyryl)phenyl)-7-oxo-bridged bicyclo[2.2.1] - Preparation of 5-heptene-2-sulfonic acid-(2-ethylphenyl) ester (14a):
[0107]
[0108] Weigh 3-(4-hydroxyphenyl)-4-(((E)-3,5-dimethoxystyryl)phenyl)furan compound 10 (300mg, 0.753mmol) and ethylene 2-ethyl Phenylsulfonate (192mg, 0.903mmol) was placed in a 25ml two-neck round-bottom flask, and 4ml of anhydrous THF was added to aid in dissolution, then the temperature was slowly raised to 90°C, and the reaction was carried out for 8 hours. TLC detected that the reaction was complete, quenched by adding water, and extracted with ethyl acetate , the organic layer was taken and dried over anhydrous sodium sulfate. Desolvation under reduced pressure, separation and purification by column chromatography, the eluent ratio is petroleum ether:ethyl acetate=5:1, 294.3 mg of brown solid was obtained, the yield was 73%, m.p.110-113°C; 1H NMR (400MHz, Acetone) δ8.80 (s, 1H), 7.5...
Embodiment 2
[0109] Example 2: 5-(4-hydroxyphenyl)-6-(4-((E)-3,5-dimethoxystyryl)phenyl)-7-oxo-bridged bicyclo[2.2.1] - Preparation of 5-heptene-2-sulfonic acid-(3-methoxyphenyl)ester (14b):
[0110]
[0111] Weigh 3-(4-hydroxyphenyl)-4-(((E)-3,5-dimethoxystyryl)phenyl)furan compound 10 (300mg, 0.753mmol) and ethylene 3-methoxy Phenylphenylsulfonate (194mg, 0.903mmol) was placed in a 25ml two-necked round-bottom flask, 4ml of anhydrous THF was added to aid dissolution, and then the temperature was slowly raised to 90°C, and the reaction was carried out for 8h. TLC detected that the reaction was complete, quenched by adding water, and ethyl acetate After extraction, the organic layer was taken and dried over anhydrous sodium sulfate. Desolvation under reduced pressure, separation and purification by column chromatography, the eluent ratio is petroleum ether:ethyl acetate=5:1, 292.8 mg of yellow solid was obtained, the yield was 63%, m.p.98-101°C. 1 H NMR (400MHz, Acetone) δ7.56(t, J=8....
Embodiment 3
[0112] Example 3: 5-(4-hydroxyphenyl)-6-(4-((E)-3,5-dimethoxystyryl)phenyl)-7-oxo-bridged bicyclo[2.2.1] - Preparation of 5-heptene-2-sulfonic acid-(4-bromophenyl) ester (14c):
[0113]
[0114] Weigh 3-(4-hydroxyphenyl)-4-(((E)-3,5-dimethoxystyryl)phenyl)furan compound 10 (300mg, 0.753mmol) and vinyl 4-bromobenzene Sulfonate (274mg, 0.903mmol) was placed in a 25ml two-necked round-bottom flask, 4ml of anhydrous THF was added to aid dissolution, and then the temperature was slowly raised to 90°C, and the reaction was carried out for 8h. TLC detected that the reaction was complete, quenched by adding water, and extracted with ethyl acetate. The organic layer was taken and dried over anhydrous sodium sulfate. Desolvation under reduced pressure, separation and purification by column chromatography, the eluent ratio is petroleum ether: ethyl acetate = 5:1, and 378.6 mg of brown solid was obtained with a yield of 76%, m.p.100-102°C. 1 H NMR (400MHz, Acetone) δ8.74(s, 1H), 7.60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com