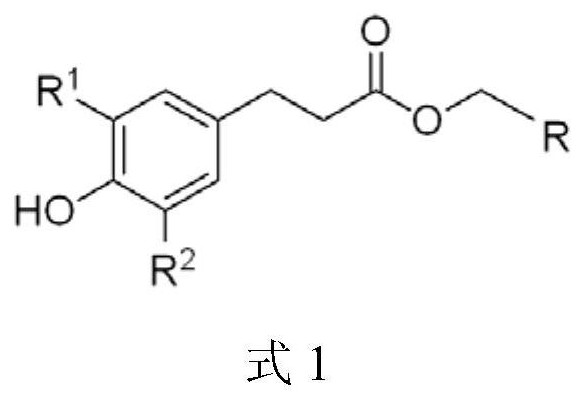
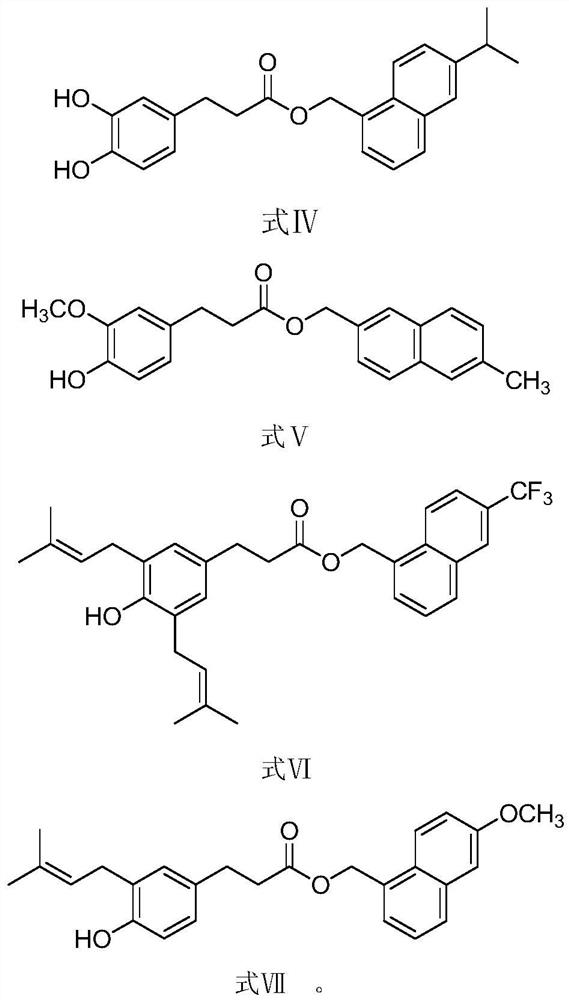
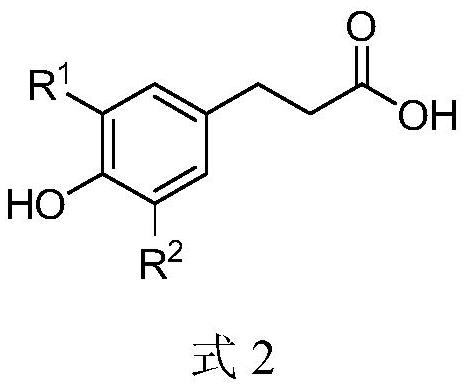
A kind of phenylpropionate derivative and its preparation method and application
A technology of phenylpropionate and derivatives, which is applied in the field of related medicines, can solve the problems of less attention on lipid-lowering effect, achieve the effects of improving lipid metabolism, optimizing synthesis route and process parameter conditions, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1 2
[0050] The synthesis of example 1 dihydrocaffeic acid-2-naphthyl methyl ester (PN1 for short, as shown in formula II)
[0051]
[0052] 1) Weigh 1.10mmol of dihydrocaffeic acid, dissolve in 1.5mL DMSO, add 1.32mmol of Na 2 CO 3 After stirring at room temperature for 0.5 h, 1.10 mmol of potassium iodide and 1.20 mmol of 2-(chloromethyl)naphthalene were added in sequence. The reaction mixture was stirred at 60 °C for 6 h (end of reaction by TLC).
[0053] 2) After the reaction was completed, cool to room temperature, add 5 mL of saturated sodium chloride solution and 5 mL of ethyl acetate, mix well and let stand to separate layers; the aqueous phase was extracted twice with ethyl acetate (3 mL×2 times), and the organic phase was combined, Then washed with saturated copper sulfate solution (3mL×4 times), and saturated sodium chloride solution (2mL×2 times), dried over anhydrous sodium sulfate, and then rotary evaporated (-0.08MPa, 45°C) to remove the solvent to obtain solid ...
example 2
[0055] The synthesis of example 2 p-hydroxyphenylpropionic acid-1-naphthylmethyl ester (PN for short, as shown in formula III)
[0056]
[0057] 1) Weigh 1.10mmol of p-hydroxyphenylpropionic acid, dissolve in 1.5mL of acetone, add 2.2mmol of NaHCO 3 After stirring at room temperature for 1 h, 2.2 mmol of lithium iodide and 2.2 mmol of 1-(bromomethyl)naphthalene were sequentially added. The reaction mixture was stirred at 40 °C for 12 h (end of reaction by TLC).
[0058] 2) Cool to room temperature after the reaction, add 10mL of saturated sodium chloride solution and 5mL of ethyl acetate, mix well and let stand to separate layers; extract the aqueous phase twice with ethyl acetate (3mL×2 times), combine the organic phases, Wash with saturated sodium chloride solution (2 mL×2 times), dry over anhydrous sodium sulfate, and then remove the solvent by rotary evaporation (-0.08 MPa, 45° C.) to obtain a solid crude product.
[0059] 3) The crude product is purified by column ch...
example 3 2
[0060] The synthesis of example 3 dihydrocaffeic acid-1-naphthalene-(5-isopropyl)-methyl ester (PN for short, as shown in formula IV)
[0061]
[0062] 1) Weigh 1.10mmol of dihydrocaffeic acid, dissolve in 1.5mL DMF, add 3.3mmol of KHCO 3 After stirring at room temperature for 1 h, 3.3 mmol of sodium iodide and 1.20 mmol of 1-(chloromethyl)-(5-isopropyl)-naphthalene were added sequentially. The reaction mixture was stirred at 80 °C for 3 h (end of reaction by TLC).
[0063] 2) After the reaction was completed, cool to room temperature, add 5 mL of saturated sodium chloride solution and 5 mL of ethyl acetate, mix well and let stand to separate layers; the aqueous phase was extracted twice with ethyl acetate (3 mL×2 times), and the organic phase was combined, Then washed with saturated copper sulfate solution (4mL×4 times), and saturated sodium chloride solution (2mL×2 times), dried over anhydrous sodium sulfate, and then rotary evaporated (-0.08MPa, 45°C) to remove the solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com