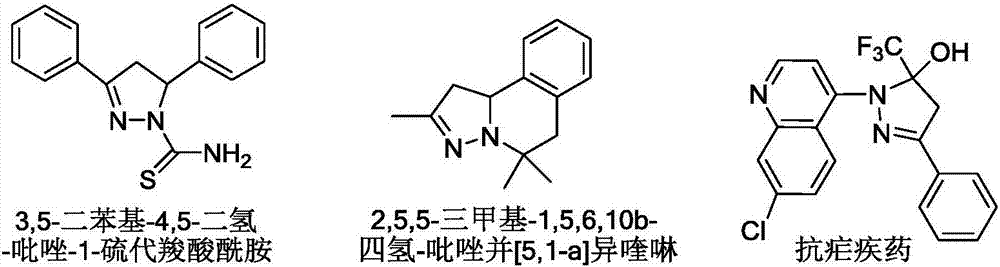
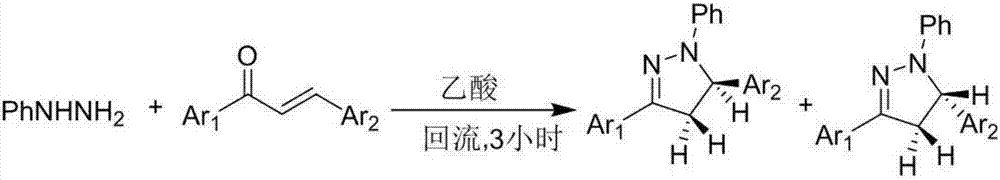
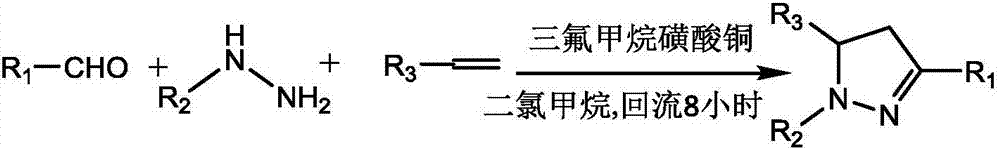
Pyrazoline derivative and preparation method thereof
A technology of dihydropyrazoles and derivatives, applied in the direction of organic chemistry, can solve the problems of expensive metal catalysts, limited application, cumbersome steps, etc., and achieve the effects of good universality, simple operation, and single target product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: The structure of 5-(azidomethyl)-3-(p-chlorotolyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole is:
[0048]
[0049] Molecular formula: C 17 h 16 ClN 5 o 2 S
[0050] Chinese name: 5-(azidomethyl)-3-(p-chlorotolyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole
[0051] English name: 5-(azidomethyl)-3-(4-chlorophenyl)-1-tosyl-4,5-dihydro-pyrazole
[0052] Molecular weight: 389.0710
[0053] Appearance: yellow solid
[0054] Melting point: 120-122°C
[0055] Proton NMR spectrum: (500MHz, CDCl 3 ):δ7.80-7.75(m,2H),7.58(dd,J=8.5,1.6Hz,2H),7.34(dd,J=8.7,2.1Hz,2H),7.29(d,J=7.9Hz, 2H), 4.05(tdd, J=10.0, 6.0, 3.1Hz, 1H), 3.88(dd, J=12.6, 6.1Hz, 1H), 3.80-3.71(m, 1H), 3.10(dt, J=10.0, 1.1Hz, 2H), 2.38(d, J=1.7Hz, 3H).
[0056] Carbon NMR spectrum: (125MHz, CDCl 3 ): δ156.91, 144.77, 136.86, 131.74, 129.73, 128.96, 128.89, 128.59, 128.21, 60.74, 54.08, 37.37, 21.64.
[0057] Infrared Absorption Spectrum ν max 3043,2925,2853,2105,1600,1450,1355,1290,1170,1090,933,...
Embodiment 2
[0059] Example 2: The structure of 5-(azidomethyl)-3-(m-chlorotolyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole is:
[0060]
[0061] Molecular formula: C 17 h 16 ClN 5 o 2 S
[0062] Chinese name: 5-(azidomethyl)-3-(m-chlorotolyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole
[0063] English name: 5-(azidomethyl)-3-(3-chlorophenyl)-1-tosyl-4,5-dihydro-pyrazole
[0064] Molecular weight: 389.0710
[0065] Appearance: yellow solid
[0066] Melting point: 108-110°C
[0067] Proton NMR spectrum: (500MHz, CDCl 3 ): δ7.79(d, J=8.3Hz, 2H), 7.65(t, J=1.9Hz, 1H), 7.51(dd, J=7.7, 1.4Hz, 1H), 7.41-7.35(m, 1H) ,7.31(t,J=7.7Hz,3H),4.14-4.02(m,1H),3.88(dd,J=12.6,6.1Hz,1H),3.76(dd,J=12.6,3.1Hz,1H), 3.17-3.05(m,2H),2.40(s,3H).
[0068] Carbon NMR spectrum: (125MHz, CDCl 3 ): δ156.59, 144.79, 134.79, 132.23, 131.82, 130.72, 129.96, 129.76, 128.58, 126.89, 125.05, 60.69, 54.09, 37.35, 21.65.
[0069] Infrared Absorption Spectrum ν max 3071,2923,2855,2100,1599,1447,1356,1291,1170,1091,9...
Embodiment 3
[0071] Example 3: The structure of 5-(azidomethyl)-3-(m-bromomethylphenyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole is:
[0072]
[0073] Molecular formula: C 17 h 16 BrN 5 o 2 S
[0074] Chinese name: 5-(azidomethyl)-3-(m-bromomethylphenyl)-1-toluenesulfonyl-4,5-dihydro-pyrazole
[0075] English name: 5-(azidomethyl)-3-(3-bromophenyl)-1-tosyl-4,5-dihydro-pyrazole
[0076] Molecular weight: 433.0206
[0077] Appearance: yellow solid
[0078] Melting point: 121-123°C
[0079] Proton NMR spectrum: (500MHz, CDCl 3 ):δ7.81-7.75(m,3H),7.58-7.49(m,2H),7.30(d,J=8.0Hz,2H),7.24(t,J=7.9Hz,1H),4.06(tdd, J=9.7,6.0,3.2Hz,1H),3.88(dd,J=12.7,6.0Hz,1H),3.74(dd,J=12.6,3.1Hz,1H),3.16-3.04(m,2H),2.39 (s,3H).
[0080] Carbon NMR spectrum: (125MHz, CDCl 3): δ156.56, 144.83, 133.63, 132.45, 131.75, 130.23, 129.78, 129.67, 128.56, 125.51, 122.83, 60.71, 54.06, 37.30, 21.65.
[0081] Infrared Absorption Spectrum ν max 3073,2923,2854,2100,1598,1451,1356,1292,1170,1090,995,854,808,770cm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com