Expression vector for enhancing gene expression of 3 beta-HSD, construction method and application thereof
A technology of gene expression and expression vector, which is applied in the field of expression vector and its construction to promote the expression of 3β-HSD gene, and can solve the problem of lack of human cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
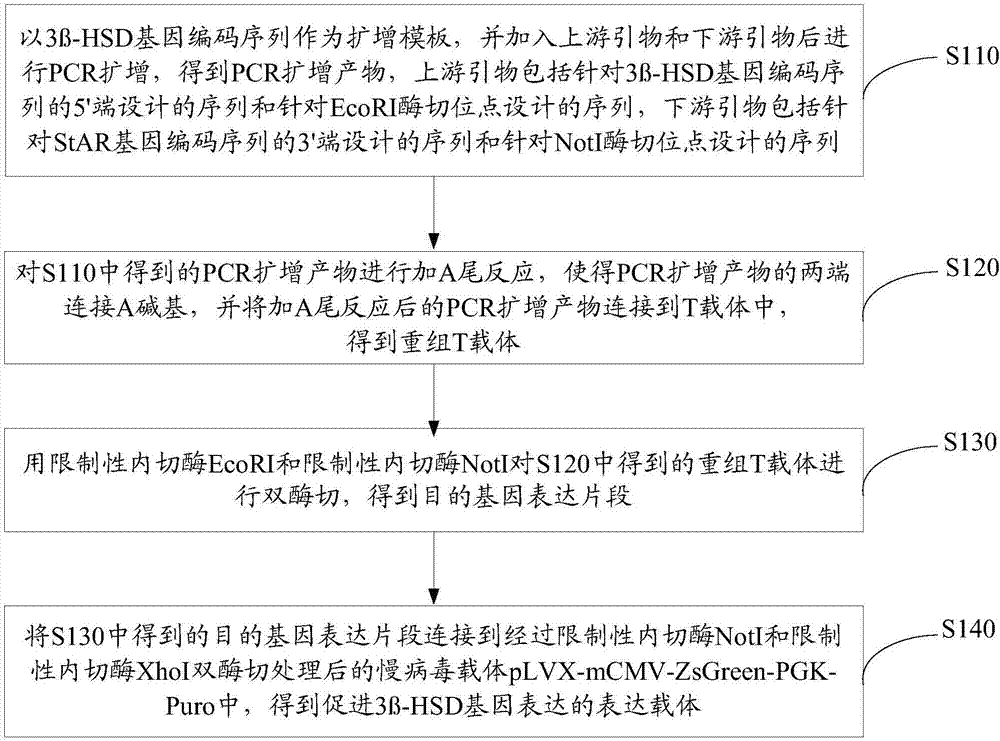
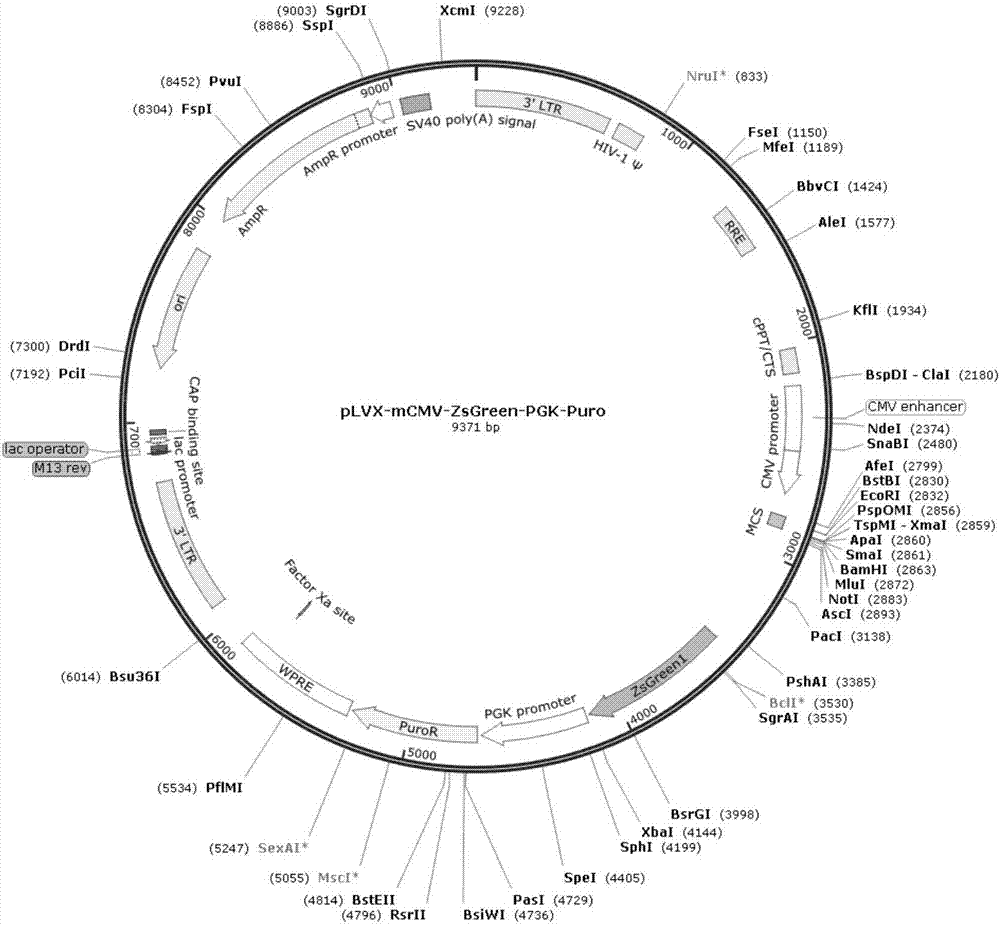
[0087] Construction of an expression vector promoting the expression of 3β-HSD gene
[0088] (1) Design of primers for 3β-HSD target gene expression fragment.
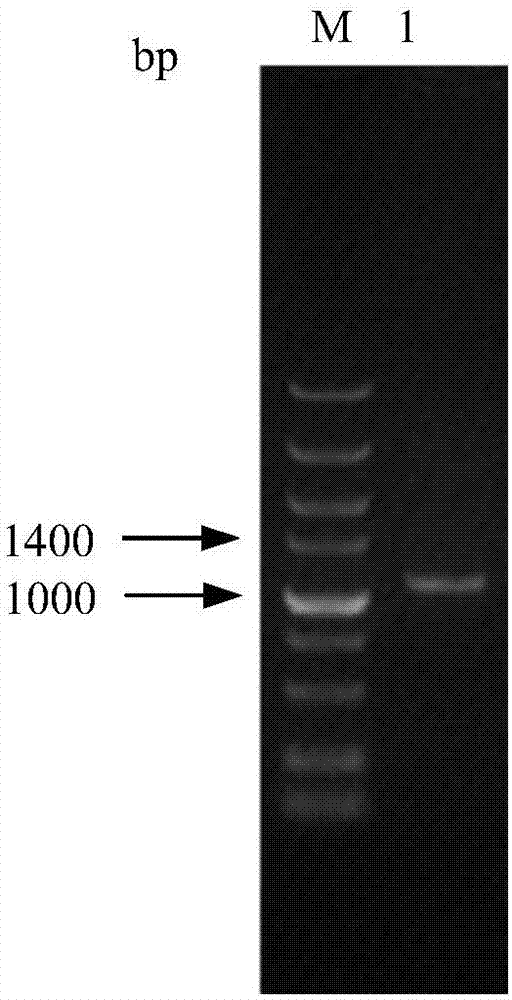
[0089] Design the 3β-HSD gene coding sequence according to the NCBI database GenBank NM_001328615, and obtain the 3β-HSD gene coding sequence (cDNA sequence of 3β-HSD) whose nucleotide sequence is shown in SEQ ID No.1. Use Oligo7 to analyze it, search for upstream and downstream primers (requires no primer dimers as much as possible and the annealing temperature difference is small), and then add protective bases and EcoR I restriction site sequences at the 5' end of the upstream primer, downstream The 5' end of the primer was added with a protective base and a Not I restriction site sequence, and the designed primer sequence is shown in Table 1. The designed PCR primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0090] Table 1: Primers for PCR amplification of the 3β-HSD gene c...
Embodiment 2
[0097] Preparation of lentivirus containing 3β-HSD gene
[0098] 293FT cells were cultured, and the cells in good growth state were inoculated into six wells, 10 per well 6 Using the lentiviral packaging auxiliary kit, transfect 2 μg of the pLVX-3β-HSD recombinant vector extracted in Example 1 into 293FT cells, culture at 37°C for 48 hours, and filter the supernatant medium with a 0.45 μM filter membrane Filter, collect the virus supernatant, use the Lenti-X GoStix Gold Label Kit to measure the virus titer, and then store it at -80°C.
Embodiment 3
[0100] Human breast cancer MCF-7 cells transduced with lentivirus
[0101] Take the virus supernatant obtained in Example 2, dilute it 1:1 with RPMI-1640 complete medium, and then add Polybrene to a final concentration of 6 μg / mL-10 μg / mL for use. will be 3×10 5 MCF-7 cells were inoculated in T25 cell culture flasks, and the confluence of the cells reached 50% after 18 hours of culture. The original complete medium in the culture flasks was removed, washed twice with PBS, and then added to the above-mentioned RPMI-1640 containing lentivirus for complete culture. base. After transfection for 24 hours, remove the RPMI-1640 complete medium containing lentivirus, add normal RPMI-1640 complete medium and culture for another 24 hours, then replace the cells with 1 μg / mL puromycin for selection, and change the medium every 2 days 1 The second time, the screening time was 7 days, and the MCF-7 cell line (pLVX-3β-HSD cell line) with high expression of 3β-HSD gene was screened.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com