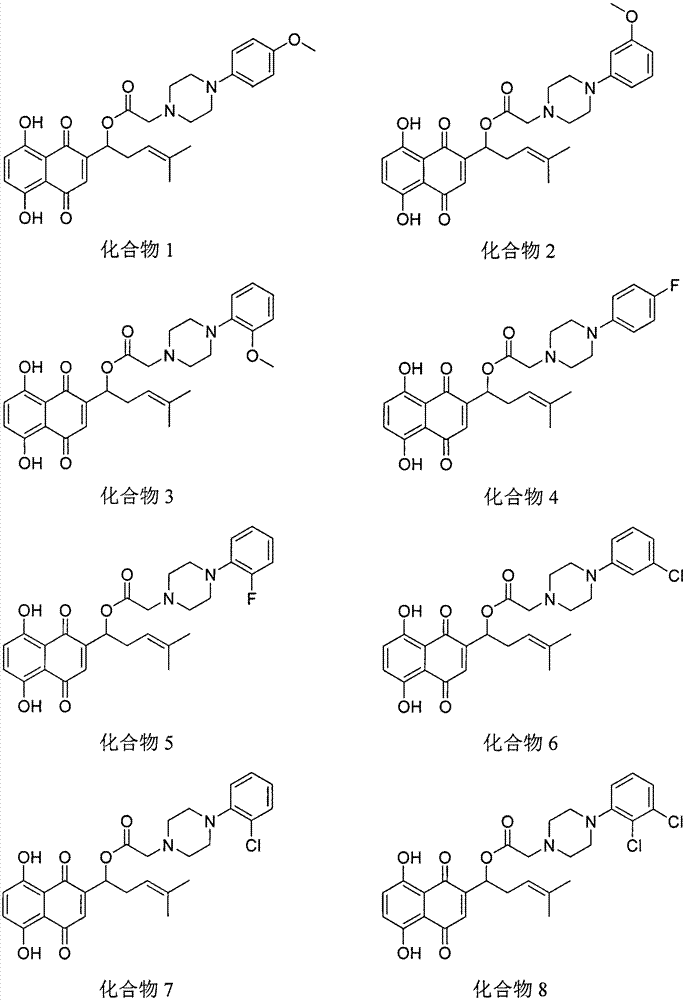
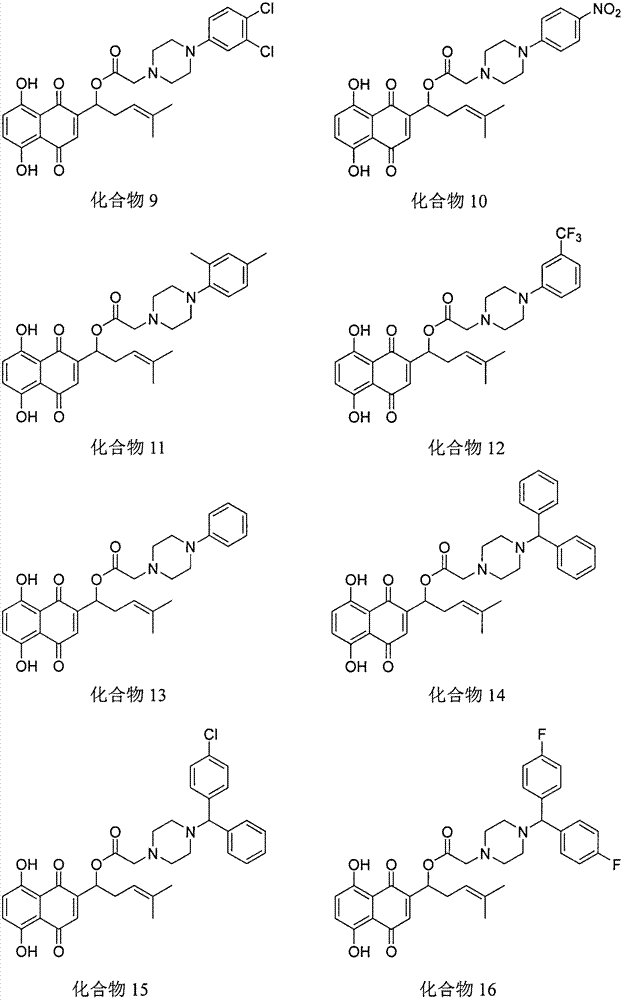
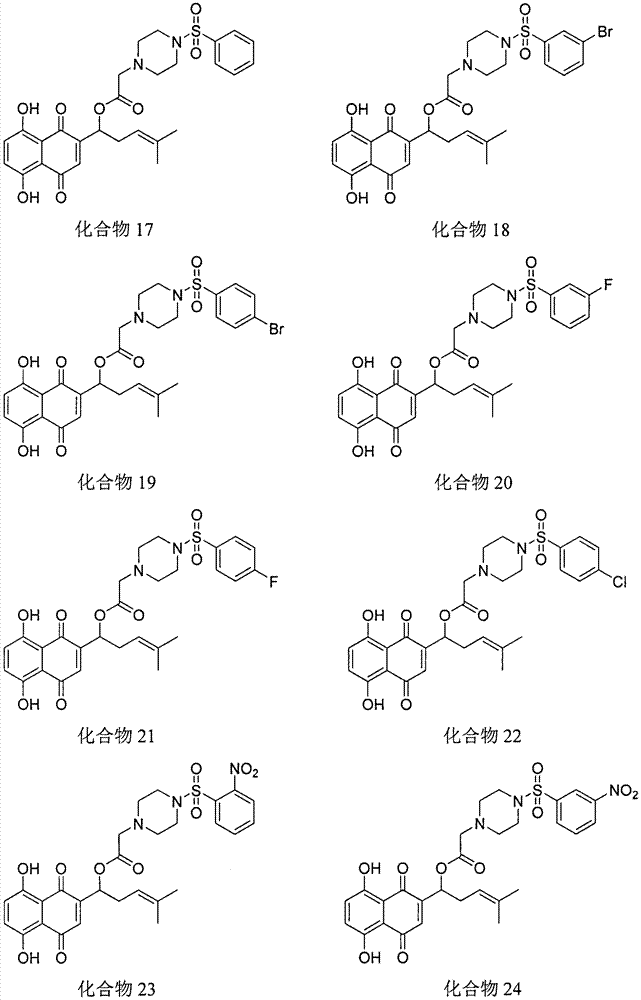
Shikonin piperazine acid ester derivatives as well as synthetic method and application thereof
A technology of shikonin piperazine acid and its derivatives, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of shikonin toxicity, side effects, low water solubility, etc., and achieve obvious antibacterial activity and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example 1: Preparation of Shikonin piperazine ester derivatives
[0016] Under the condition of ice bath, add shikonin, corresponding carboxylic acid, refined dichloromethane and catalyst in sequence in a 50mL round bottom flask, TLC detects that the reaction is complete, and separates by thin plate chromatography to obtain the corresponding shikonin piperazinate derivatives .
[0017] The physicochemical data of the corresponding compounds are as follows:
[0018] Compound 1: yield 61%; 1 H NMR (300MHz, CDCl 3 )δ12.58(s, 1H), 12.39(s, 1H), 7.18(s, 2H), 7.15-7.08(m, 1H), 7.08-6.94(m, 2H), 6.92(d, J=8.8Hz , 2H), 6.12(dd, J=6.9, 4.9Hz, 1H), 5.10(t, J=7.2Hz, 1H), 3.80(s, 3H), 3.56(s, 2H), 3.48(s, 4H) , 3.27(s, 4H), 2.69-2.60(m, 1H), 2.58-2.50(m, 1H), 1.69(s, 3H), 1.59(s, 3H). MS(ESI): 521.2([M+ H] + ).
[0019] Compound 2: yield 63%; 1 H NMR (300MHz, CDCl 3 )δ12.57(s, 1H), 12.36(s, 1H), 7.26(d, J=16.3Hz, 1H), 7.16(s, 2H), 7.10(s, 1H), 6.82(s, 2H), 6.67(d, J=7.6Hz...
example 2
[0050] Example 2: Application of Formula I Shikonin Carboxylate Derivatives
[0051] Gram-positive bacteria B.subtilis, S.aureus and Gram-negative bacteria E.coli, P.aeruginosa strains were used as detection strains, and MTT colorimetry was used as the detection method, through the detection of formula I class shikonin piperazine acid The research on the antibacterial activity of ester derivatives found that this kind of novel structural derivatives has obvious antiproliferative activity against Gram-positive bacteria. The results are shown in Table 1.
[0052] The shikonin carboxylate derivatives of the present invention can be prepared into antibacterial drugs.
[0053] Table 1 Antibacterial activity of shikonin carboxylate derivatives
[0054]
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com