Para aminobenzoyl chitosan and preparing method thereof
A technology of aminobenzoyl chitosan and p-aminobenzoic acid, applied in the field of polymer chemistry, can solve the problem of chitosan being insoluble in water and the like, achieve good antibacterial activity and expand the effect of antibacterial range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take 1 g of dried p-aminobenzoic acid and put it into a three-necked bottle, add 30 ml of anhydrous ether, then add 2 ml of thionyl chloride, put it into an ultrasonic scrubber with a water temperature of 30°C, and react for 10 minutes. Cool to 25°C, dissolve 0.5g of chitosan in 30ml of acetic acid, add it into a three-necked bottle, vibrate ultrasonically for 1 hour at 25°C, and let stand overnight. After the reaction was completed, add 70ml of acetone to precipitate the product, filter it with suction, then soak it in a mixed solvent of absolute ethanol and ether with a volume ratio of 1:1 with 50ml and then filter it with suction after 24 hours. Soxhlet extraction was carried out with acetone for 24 hours, and the product—p-aminobenzoyl chitosan was obtained after vacuum drying.
Embodiment 2
[0040] Take 1 g of dried p-aminobenzoic acid and put it into a three-necked bottle, add 30 ml of anhydrous ether, then add 2 ml of thionyl chloride, put it into an ultrasonic scrubber with a water temperature of 30°C, and react for 10 minutes. Heating to 45°C, dissolving 0.5g of chitosan in 30ml of acetic acid, adding it into a three-necked bottle, ultrasonically oscillating at 45°C for 1 hour, and standing overnight. After the reaction was completed, add 70ml of acetone to precipitate the product, filter it with suction, then soak it in a mixed solvent of absolute ethanol and ether with a volume ratio of 1:1 with 50ml and then filter it with suction after 24 hours. Soxhlet extraction was carried out with acetone for 24 hours, and the product—p-aminobenzoyl chitosan was obtained after vacuum drying.
Embodiment 3
[0042] Take 1 g of dried p-aminobenzoic acid and put it into a three-necked bottle, add 30 ml of anhydrous ether, then add 2 ml of thionyl chloride, put it into an ultrasonic scrubber with a water temperature of 30°C, and react for 10 minutes. Cool to 5°C, dissolve 0.5g of chitosan in 30ml of acetic acid, add it into a three-necked bottle, vibrate ultrasonically for 1 hour at 5°C, and let stand overnight. After the reaction was completed, add 70ml of acetone to precipitate the product, filter it with suction, then soak it in a mixed solvent of absolute ethanol and ether with a volume ratio of 1:1 with 50ml and then filter it with suction after 24 hours. Soxhlet extraction was carried out with acetone for 24 hours, and the product—p-aminobenzoyl chitosan was obtained after vacuum drying.
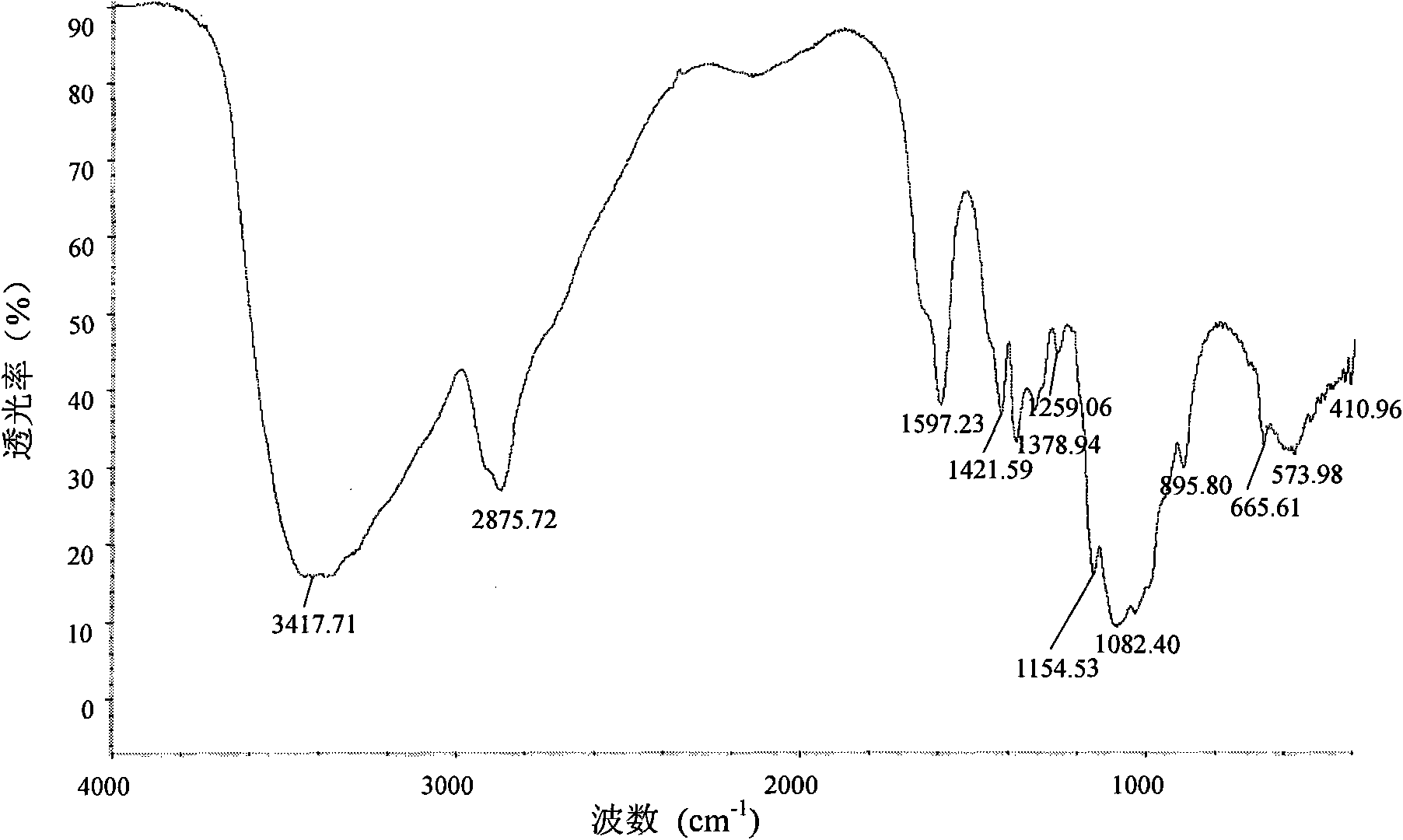
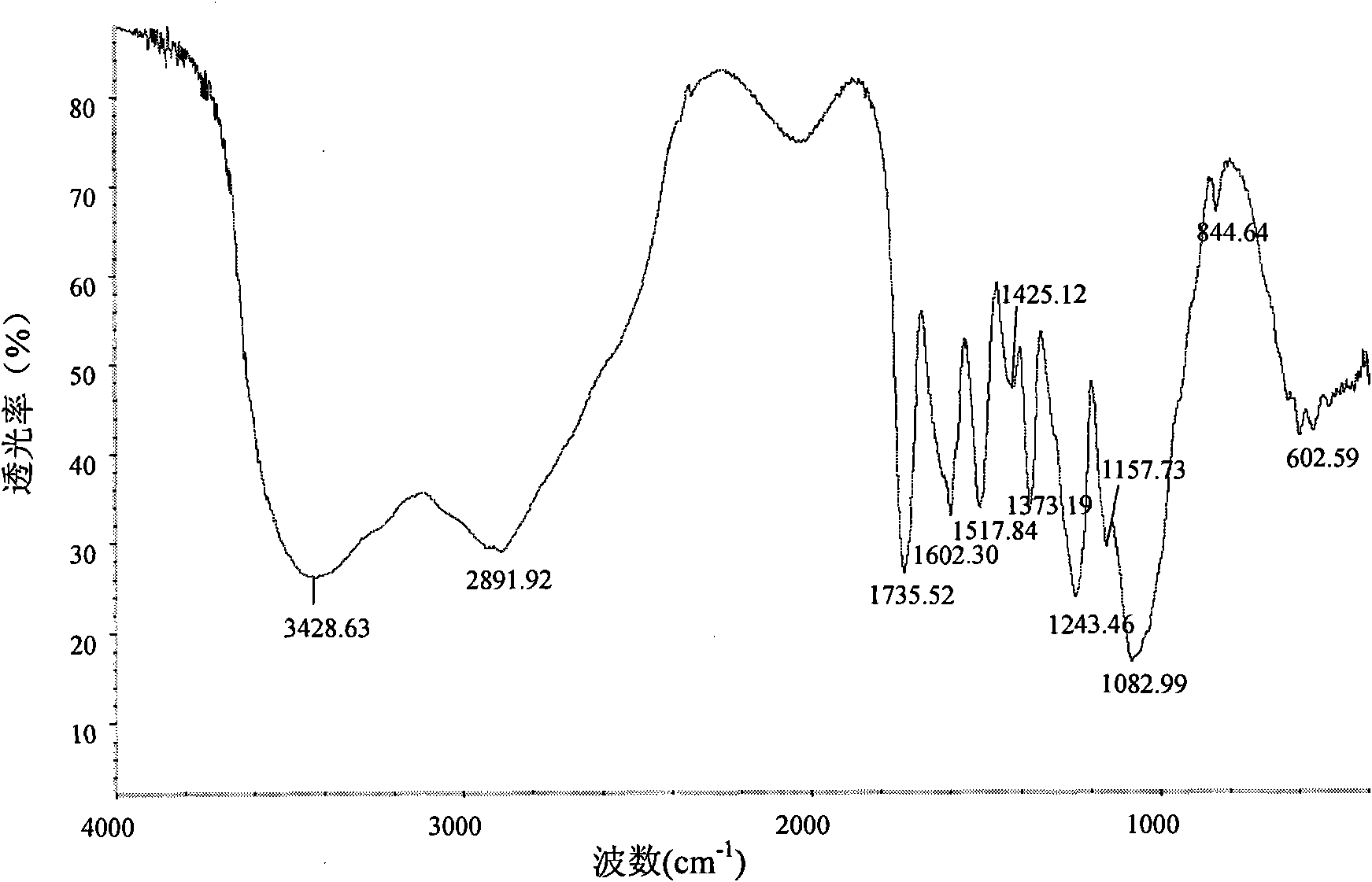
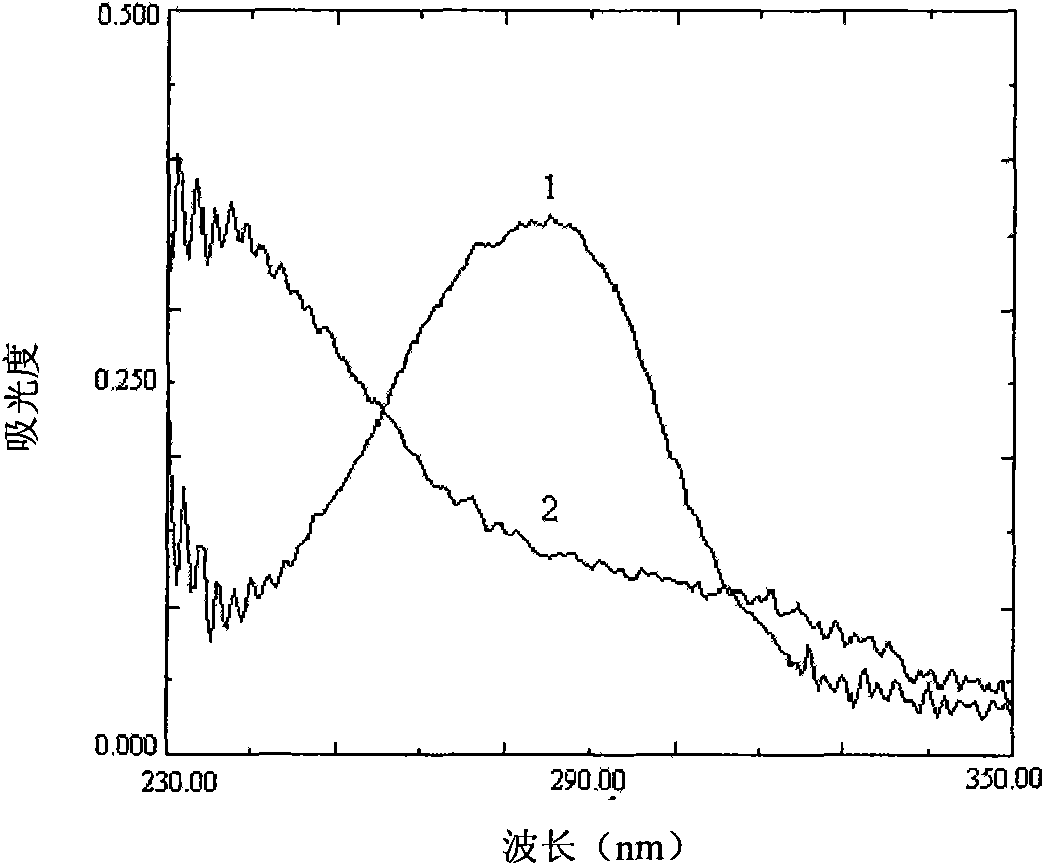
[0043] analyze figure 1 and figure 2 Two infrared spectra can be seen, chitosan infrared spectrum 1597.2cm -1 The place is the amino N-H deformation vibration peak, 2875.7cm -1 At the C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com