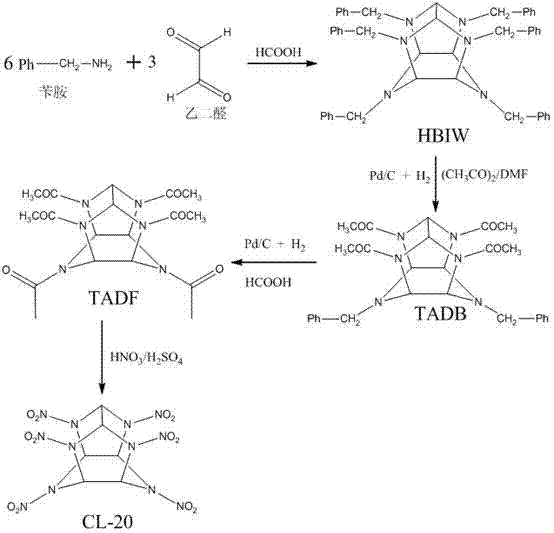
Method for preparing CL-20 through three-step reaction
A CL-20, reaction technology, applied in the direction of organic chemistry, can solve the problems of increasing the complexity of the process, and achieve the effect of simplifying the operation, shortening the process flow and reducing the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Add 75g of HBIW, 300mL of DMF, and 2.50mL of bromobenzene into a 1000mL autoclave. 3.75g Pd / C (10% metal palladium content on a dry basis), 200mL acetic anhydride. The reactor was replaced three times with nitrogen and three times with hydrogen. During the replacement process, the temperature of the reactor jacket was controlled below 10°C. After the replacement, the hydrogen pressure in the reactor rose to 0.2 MPa, and the stirring was started. As the hydrogenation reaction proceeds, the temperature of the reactor rises. Control the reaction temperature in the kettle at 28-50°C by adjusting the temperature of the jacket circulating liquid, add 240mL formic acid (85%) after 2 hours, control the reaction temperature in the kettle at 50-90°C, continue the reaction for 3 hours, and end the reaction after the reaction . Turn off the hydrogen. 33.61 g of TADF was obtained with a yield of 84%.
[0031] Synthesis of CL-20: Add 125mL of fuming sulfuric acid dropwise to 300...
Embodiment 2
[0033] Add 75g of HBIW, 300mL of DMAc (N,N-dimethylacetamide), and 2.50 mL of bromobenzene into a 1000mL autoclave. 5.0 g Pd / C (10% metal palladium content on a dry basis), 200 mL acetic anhydride. The reactor was replaced three times with nitrogen and three times with hydrogen. During the replacement process, the temperature of the reactor jacket was controlled below 10°C. After the replacement, the hydrogen pressure in the reactor rose to 0.35MPa, and the stirring was started. As the hydrogenation reaction proceeds, the temperature of the reactor rises. Control the reaction temperature in the kettle at 28-50°C by adjusting the temperature of the jacket circulating liquid, add 240mL formic acid (55%) after 3 hours, control the reaction temperature in the kettle at 50-90°C, continue the reaction for 3 hours, and end the reaction after the reaction . Turn off the hydrogen. Obtained TADF 34.86g, yield 81%.
[0034] Synthesis of CL-20: Add 125mL of fuming sulfuric acid drop...
Embodiment 3
[0036] Add 75g of HBIW, 300mL of DMAc (N,N-dimethylacetamide), and 2.50mL of bromobenzene into a 1000mL autoclave. 6.0 g Pd / C (10% palladium content on dry basis), 160 mL acetic anhydride. The reactor was replaced three times with nitrogen and three times with hydrogen. During the replacement process, the temperature of the reactor jacket was controlled below 10°C. After the replacement, the hydrogen pressure in the reactor rose to 0.25MPa, and the stirring was started. As the hydrogenation reaction proceeds, the temperature of the reactor rises. Control the reaction temperature in the kettle at 28-50°C by adjusting the temperature of the jacket circulating liquid, add 200 mL of formic acid (55%) after 2.5 hours, control the reaction temperature in the kettle at 40-90°C, continue the reaction for 3 hours, and end after the reaction reaction. Turn off the hydrogen. TADF 31.38 was obtained with a yield of 75.60%.
[0037] Synthesis of CL-20: Add 125mL of fuming sulfuric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com