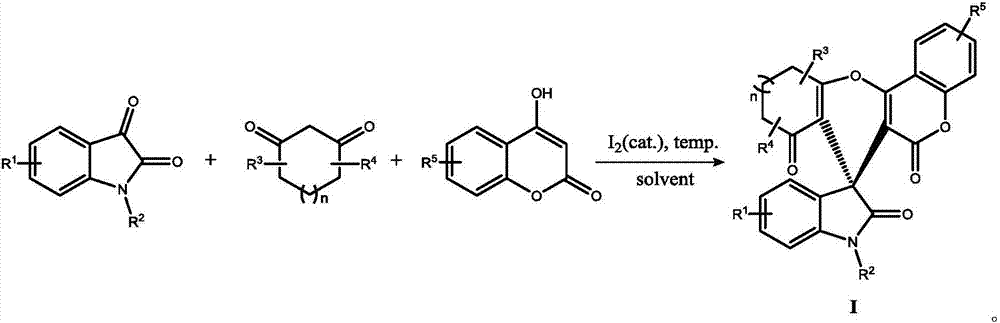
Method for preparing indolyl spirocyclic compound through iodine catalyzed multi-component reaction
A multi-component reaction, indole spiro ring technology, applied in the direction of organic chemistry, can solve the problems of polluted environment, corroded equipment, many side reactions, etc., to achieve the effects of simple reaction operation, high catalytic efficiency, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
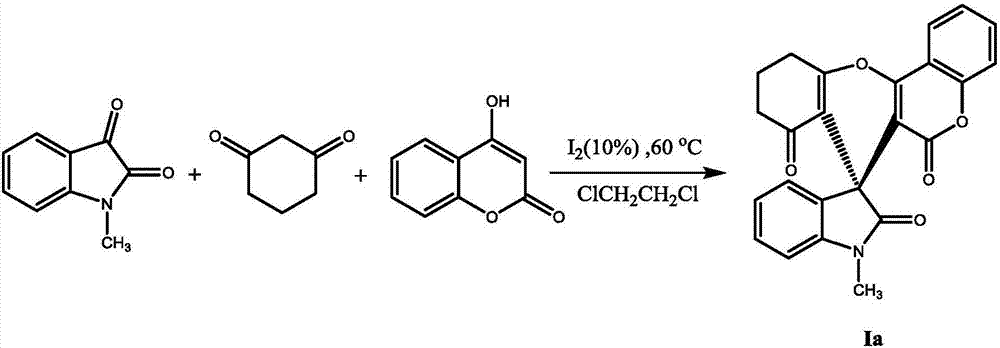
Embodiment 1
[0018]
[0019] Accurately weigh 0.1611g N-methylisatin, 0.1121g 1,3-cyclohexanedione, 0.1621g 4-hydroxycoumarin and 0.0254gI 2 Dissolve in 4mL ClCH 2 CH 2 After magnetic stirring at 60° C. for 24 h in Cl, the reaction was determined to be complete by TLC detection, the solvent was removed by rotary evaporation, and a white solid Ia was obtained by column chromatography. Yield 82%, m.p.320.5~321.2℃; 1 HNMR (400MHz, CDCl 3 )δ: 7.88(d, J=7.9Hz, 1H), 7.61~7.56(m, 1H), 7.36(t, J=7.6Hz, 1H), 7.28(d, J=8.1Hz, 2H), 6.96~ 6.92(m,2H),6.88(d,J=7.7Hz,1H),3.38(s,3H),2.86~2.83(m,2H),2.46~2.31(m,2H),2.18~2.02(m, 2H); 13 C NMR (100MHz, CDCl 3 )δ: 195.1, 176.7, 164.9, 158.5, 154.8, 152.6, 145.6, 132.9, 132.1, 129.1, 124.4, 122.7, 122.6, 122.1, 116.8, 114.6, 113.1, 107.9, 106.5, 40.1, 20.0, 397.2 .Anal.Calcd for C 24 h 17 NO 5 : C 72.17, H 4.29, N 3.51; Found: C 72.37, H 4.51, N 3.40.
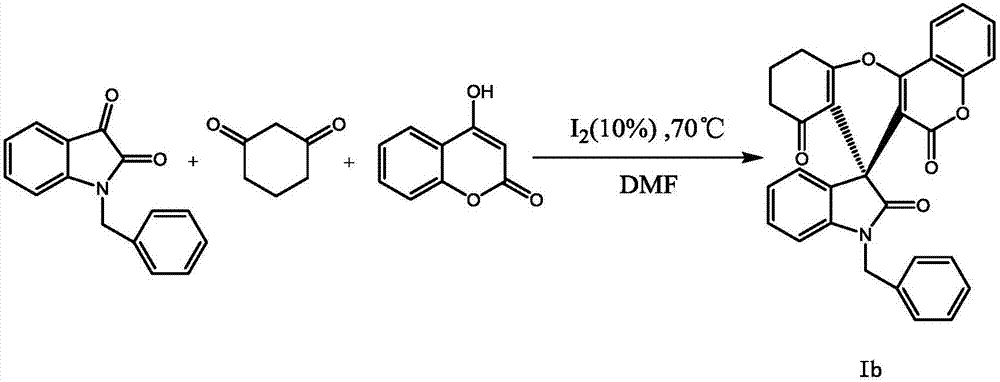
Embodiment 2
[0021]
[0022] Accurately weigh 0.2373g N-benzyl isatin, 0.1121g 1,3-cyclohexanedione, 0.1621g 4-hydroxycoumarin and 0.0254gI 2 Dissolved in 4 mL of DMF, stirred magnetically at 70°C for 36 hours, detected by TLC, confirmed that the reaction had ended, removed the solvent by rotary evaporation, and obtained white solid Ib by column chromatography, with a yield of 83%, m.p.306.9-307.1°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 8.04(d, J=7.8, Hz, 1H), 7.76(t, J=7.8Hz, 1H), 7.63(d, J=7.5Hz, 2H), 7.52(t, J=7.7Hz, 1H ),7.47(d,J=8.2Hz,1H),7.36(t,J=7.5Hz,2H),7.28(t,J=7.3Hz,1H),7.12(t,J=8.1Hz,1H), 6.88(t, J=7.4Hz, 1H), 6.64(d, J=7.8Hz, 1H), 4.97(ABd, J=16.4Hz, 1H), 4.92(ABd, J=16.4Hz, 1H), 2.96~ 2.93(m,2H),2.42~2.26(m,2H),2.05~1.95(m,2H); 13 C NMR (100MHz, DMSO-d 6 )δ: 195.8, 176.8, 166.2, 158.6, 155.1, 152.3, 145.2, 137.0, 134.1, 132.6, 128.9, 128.8, 127.8, 127.5, 125.5, 123.9, 123.6, 122.4, 117.0, 113.9, 419.5, 18012 ,44.7,37.3,27.4,20.1.Anal.Calcd forC 30 h 21 NO 5 : C 75.78, H 4...
Embodiment 3
[0024]
[0025] Accurately weigh 0.2401g 5-bromo-N-methylisatin, 0.0981g 1,3-cyclopentanedione, 0.1621g 4-hydroxycoumarin and 0.0508g I 2 Dissolve in 4mL CH 2 Cl 2 In the process, after magnetic stirring at 60°C for 24 hours, TLC detection confirmed that the reaction had ended, the solvent was removed by rotary evaporation, and a white solid Ic was obtained by column chromatography with a yield of 79%, m.p.315.1-315.4°C; 1 HNMR (400MHz, DMSO-d 6 )δ:8.07(dd,J=7.9,1.3Hz,1H),7.82~7.77(m,1H),7.57~7.52(m,2H),7.50~7.47(m,2H),7.05(d,J= 8.8Hz, 1H), 3.21(s, 3H), 3.03~3.00(m, 2H), 2.50~2.47(m, 2H); 13 C NMR (100MHz, DMSO-d 6 )δ: 200.5, 177.6, 174.7, 159.2, 157.6, 152.5, 144.5, 134.3, 133.6, 132.1, 127.3, 125.6, 123.6, 117.1, 116.5, 114.5, 113.3, 110.4, 105.3, 46.1, 27.1, 33.1 .Calcd for C 23 h 14 BrNO 5 : C 59.50, H 3.04, N 3.02; Found: C 59.56, H 3.03, N 2.81.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com