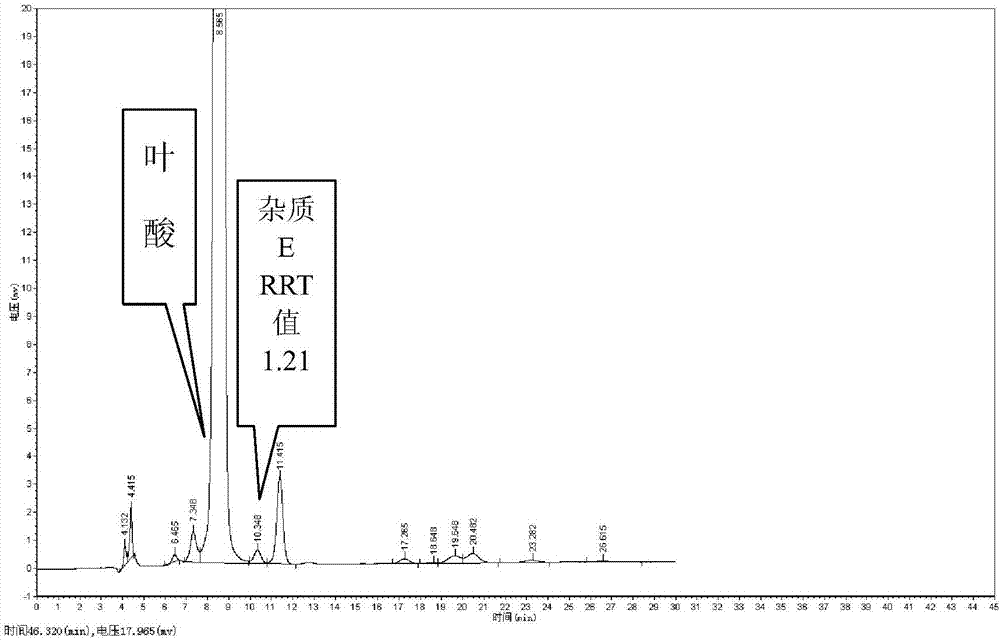
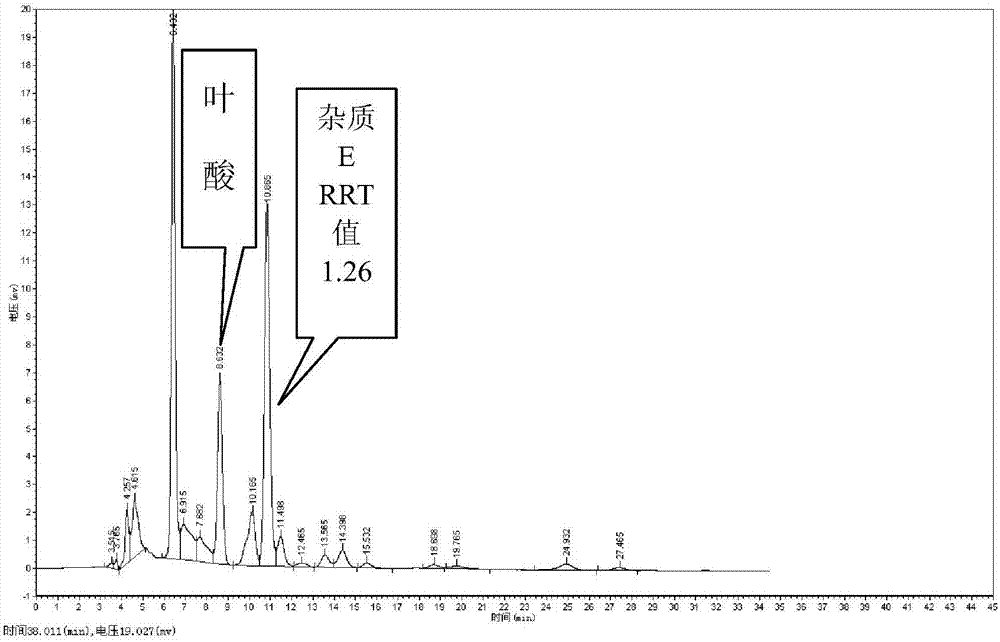
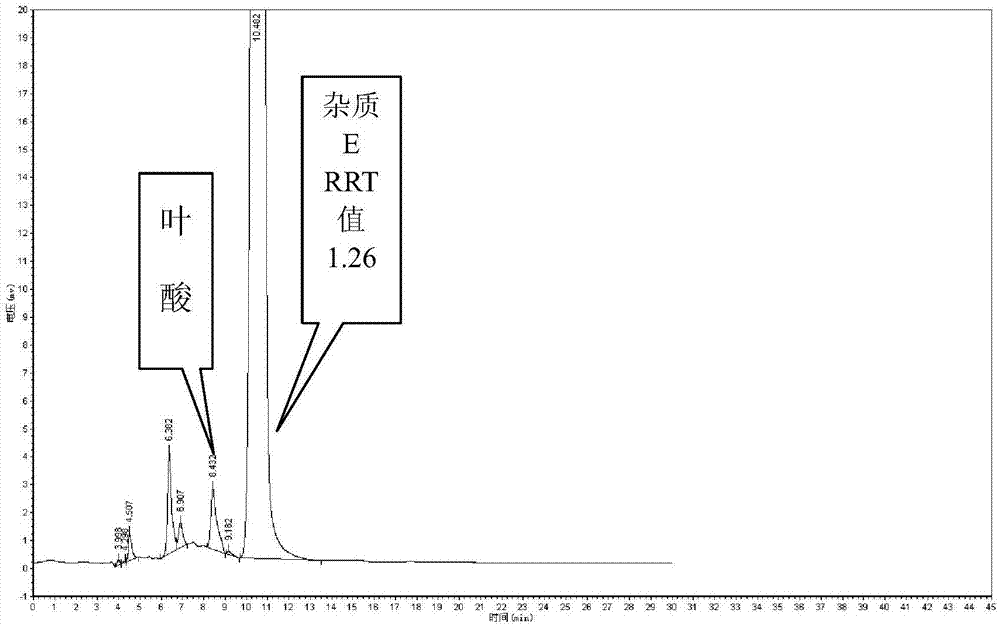
Preparation method and application of pteridine folic acid
A technology of pteridine folic acid and sulfate, which is applied in the preparation of test samples, organic chemistry and other directions, can solve the problems such as the production mechanism and control method of finished folic acid products that have not been studied in depth, and achieve the effect of improving the quality control level of folic acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Synthesis of crude pteridine folic acid
[0044] Dissolve p-aminobenzoyl glutamic acid, triaminopyrimidine sulfate, and trichloroacetone in water. Based on the mass ratio, p-aminobenzoyl glutamic acid: triaminopyrimidine sulfate: trichloroacetone: water = 1:2:3:100, control the pH of the reaction solution to 1, heat to 80°C, keep it warm for 5 hours, cool to 20°C, filter, adjust the pH of the filtrate to 5 with NaOH, and filter to obtain a crude product with a purity of 26.23%;
[0045] (2) Purification of pteridine folic acid
[0046] The crude product is dissolved in ammonia water, filtered, and the filtrate enters the semi-preparative chromatography, 10ml each time, collects the effluent, merges and concentrates under reduced pressure, the concentrate is diluted with purified water twice, and then enters the semi-preparative chromatography, washed with 10% mass fraction of methanol The effluent is collected, concentrated and freeze-dried to obtain pteridine folic acid....
Embodiment 2
[0052] (1) Synthesis of crude pteridine folic acid
[0053] Dissolve p-aminobenzoyl glutamic acid, triaminopyrimidine sulfate, and trichloroacetone in water. Based on the mass ratio, p-aminobenzoyl glutamic acid: triaminopyrimidine sulfate: trichloroacetone: water = 1:3:4:120, control the pH of the reaction solution to 2, heat to 90°C, keep for 8 hours, lower the temperature to 30°C, filter, adjust the pH of the filtrate to 3 with NaOH, and filter to obtain a crude product with a purity of 22.51%;
[0054] (2) Purification of pteridine folic acid
[0055] The crude product is dissolved in ammonia water, filtered, and the filtrate enters the semi-preparative chromatography, 10 ml each time, collects the effluent, merges and concentrates under reduced pressure, the concentrate is diluted with purified water twice, and then enters the semi-preparative chromatography, washed with 10% methanol. The effluent is collected, concentrated and freeze-dried to obtain pteridine folic acid, the c...
Embodiment 3
[0058] (1) Synthesis of crude pteridine folic acid
[0059] Dissolve p-aminobenzoyl glutamic acid, triaminopyrimidine sulfate, and trichloroacetone in water. Based on the mass ratio, p-aminobenzoyl glutamic acid: triaminopyrimidine sulfate: trichloroacetone: water = 1:3:5:150, control the pH of the reaction solution to 2.5, heat to 93°C, keep for 9 hours, lower the temperature to 35°C, filter, adjust the pH of the filtrate to 4 with NaOH, and filter to obtain a crude product with a purity of 18.47%;
[0060] (2) Purification of pteridine folic acid
[0061] The crude product is dissolved in ammonia water, filtered, and the filtrate enters the semi-preparative chromatography, 10ml each time, collects the effluent, merges and concentrates under reduced pressure, the concentrate is diluted 4 times with purified water, and then enters the semi-preparative chromatography, washed with 20% methanol by mass fraction The effluent is collected and concentrated and freeze-dried to obtain pteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com