Aqueous metal ion pentazole salts and preparation method thereof
A technology of metal ions and pentazolium salts, which is applied in the field of pentazolium anion salts and their synthesis, can solve the problems of no progress and achieve the effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
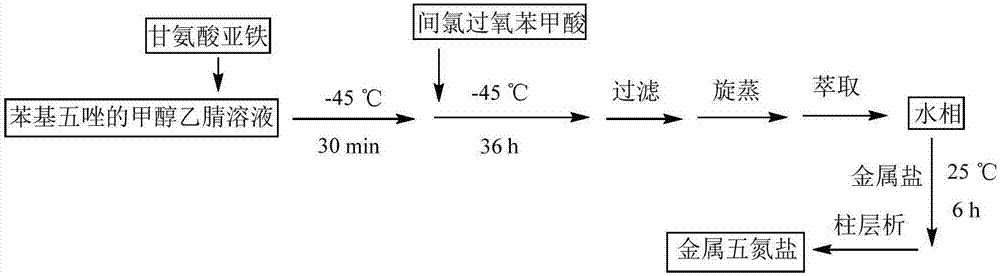
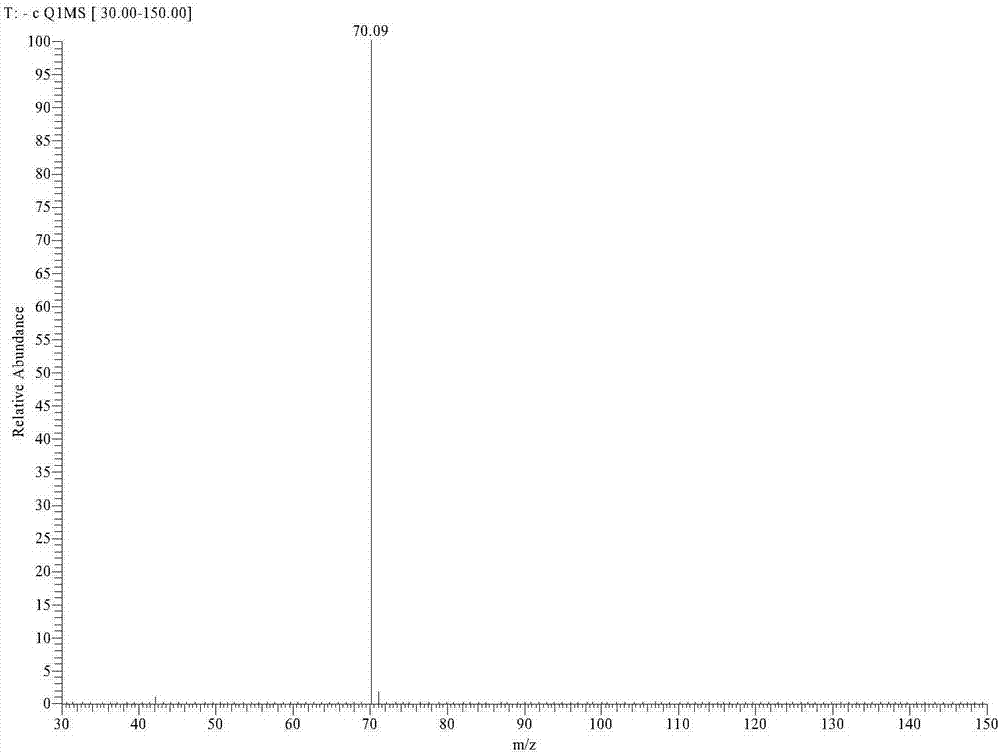
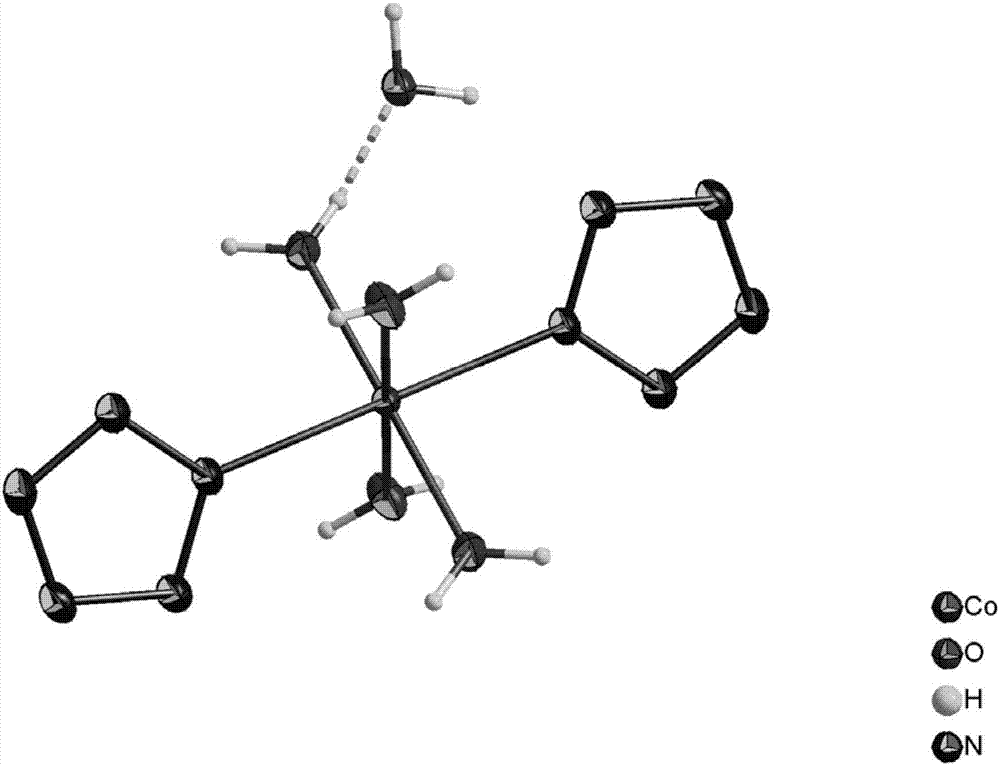
[0037] Example 1: Dissolve 3,5-dimethyl-4-hydroxyphenylpentazole (3 mmol) in a mixed solvent of methanol and acetonitrile in a single-necked flask, and add ferrous glycinate under the reaction condition of -45 °C (6 mmol) in methanol aqueous solution (pre-cooled to -45 °C), stirred for 30 min, then added m-chloroperoxybenzoic acid (Wt85%) (12 mmol) in methanol solution (pre-cooled to -45 °C), and reacted for 36 h. Filtrate, remove most of the solvent by rotary evaporation, extract by separation, combine the aqueous phases, then add cobalt chloride hexahydrate (3 mmol), stir and react at 25 °C for 3 h, and spin through the column to obtain 58.4 mg pentazole metal salt Co(N 5 )2 (H 2 O) 8 , yield 11.35%.
Embodiment example 2
[0038] Example 2: Dissolve 3,5-dimethyl-4-hydroxyphenylpentazole (3 mmol) in a mixed solvent of methanol and acetonitrile in a single-necked flask, and add ferrous glycinate under the reaction condition of -45 °C (6 mmol) in methanol aqueous solution (pre-cooled to -45 °C), stirred for 30 min, then added m-chloroperoxybenzoic acid (Wt85%) (12 mmol) in methanol solution (pre-cooled to -45 °C), and reacted for 36 h. Filtration, rotary evaporation to remove most of the solvent, liquid separation extraction, combined water phase, then added cobalt nitrate hexahydrate (6 mmol), stirred at 25 °C for 6 h, rotary evaporation through the column to obtain 63.1 mg pentazole metal salt Co(N 5 ) 2 (H 2 O) 8 , yield 12.26%.
Embodiment example 3
[0039] Example 3: Dissolve 3,5-dimethyl-4-hydroxyphenylpentazole (3 mmol) in a mixed solvent of methanol and acetonitrile in a single-necked flask, and add ferrous glycinate under the reaction condition of -45 °C (6 mmol) in methanol aqueous solution (pre-cooled to -45 °C), stirred for 30 min, then added m-chloroperoxybenzoic acid (Wt85%) (12 mmol) in methanol solution (pre-cooled to -45 °C), and reacted for 36 h. Filtration, rotary evaporation to remove most of the solvent, liquid separation extraction, combined water phase, then added cobalt sulfate heptahydrate (9 mmol), stirred at 30 °C for 6 h, rotary evaporation through the column to obtain 56.4 mg pentazole metal salt Co(N 5 ) 2 (H 2 O) 8 , yield 10.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com