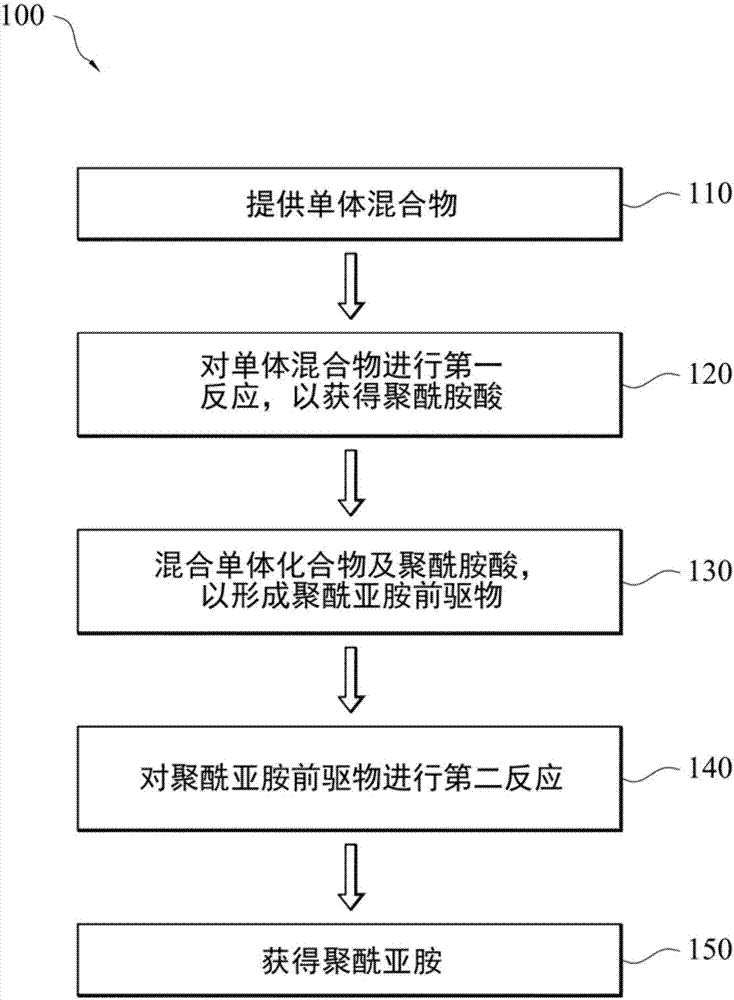
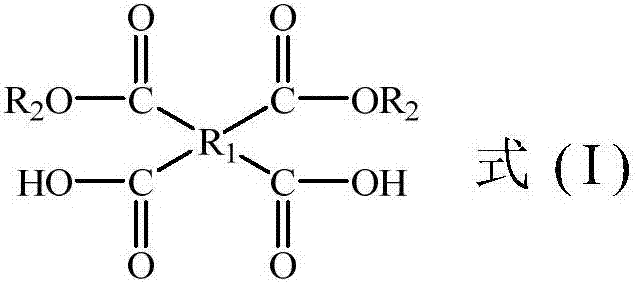
Polyimide precursor composition, preparation method of polyimide and polyimide
A technology of polyimide precursor and compound composition, which is applied in the direction of coating, etc., can solve the problems of polyimide film mechanical properties reduction, time-consuming and cost-intensive, unable to meet the needs of applications, etc., to achieve the suppression of viscosity increase, easy processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0087] In a 1000 ml four-port reactor with nitrogen, add 19.630 g (0.182 mol) of p-phenylenediamine, 2.639 g (0.0075 mol) of 9,9'-bis(4-aminophenyl)fluorene and 425 gram of N-methyl-2-pyrrolidone, and mixed uniformly at 20°C to 30°C to form a diamine compound solution.
[0088] Then, 7.434 g (0.034 mol) of pyromellitic dianhydride was added to the diamine compound solution to perform a preliminary reaction to form a reacted solution. Next, divide 44.565 grams (0.151 moles) of 3,3',4,4'-diphenyltetracarboxylic dianhydride into three equal parts, and add it to the aforementioned solution by adding in batches , and keep stirring to carry out the reaction.
[0089] After 4 hours, the polyamic acid of Synthesis Example 1 was obtained.
Synthetic example 2
[0091] In a 1000 ml four-port reactor with nitrogen gas, 26.112 grams (0.242 moles) of p-phenylenediamine, 3.511 grams (0.01 moles) of 9,9'-bis(4-aminophenyl)fluorene and 400 gram of N-methyl-2-pyrrolidone, and mixed uniformly at 20°C to 30°C to form a diamine compound solution.
[0092] Then, 9.888 g (0.045 mol) of pyromellitic dianhydride was added to this diamine compound solution to perform a preliminary reaction to form a reacted solution. Next, divide 58.54 grams (0.199 moles) of 3,3',4,4'-diphenyltetracarboxylic dianhydride into three equal parts, and add it to the aforementioned solution by adding in batches , and keep stirring to carry out the reaction.
[0093] After 4 hours, the polyamic acid of Synthesis Example 2 was obtained.
Synthetic example 3
[0095] In a 1000 ml four-port reactor with nitrogen gas, add 32.491 g (0.3 mol) of p-phenylenediamine, 4.368 g (0.013 mol) of 9,9'-bis(4-aminophenyl)fluorene and 375 gram of N-methyl-2-pyrrolidone, and mixed uniformly at 20°C to 30°C to form a diamine compound solution.
[0096] Then, 12.304 g (0.056 mol) of pyromellitic dianhydride was added to this diamine compound solution to perform a preliminary reaction to form a reacted solution. Next, divide 70.995 grams (0.241 moles) of 3,3',4,4'-diphenyltetracarboxylic dianhydride into three equal parts, and add it to the aforementioned solution by adding in batches , and keep stirring to carry out the reaction.
[0097] After 4 hours, the polyamic acid of Synthesis Example 3 was obtained.
[0098] Preparation of polyimide precursor composition
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com