Process for the preparation of oxysulfides and fluorinated compounds
A technology of fluorinated compounds and oxysulfides, applied in the direction of organic chemistry, etc., can solve problems such as limiting the recycling and reuse of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0085] Example 1: According to the continuous implementation at SO 2 Preparation of trifluoromethane by sulfination of bromotrifluoromethane in the presence of potassium hydroxide Potassium Methanesulfinate CF 3 SO 2 K
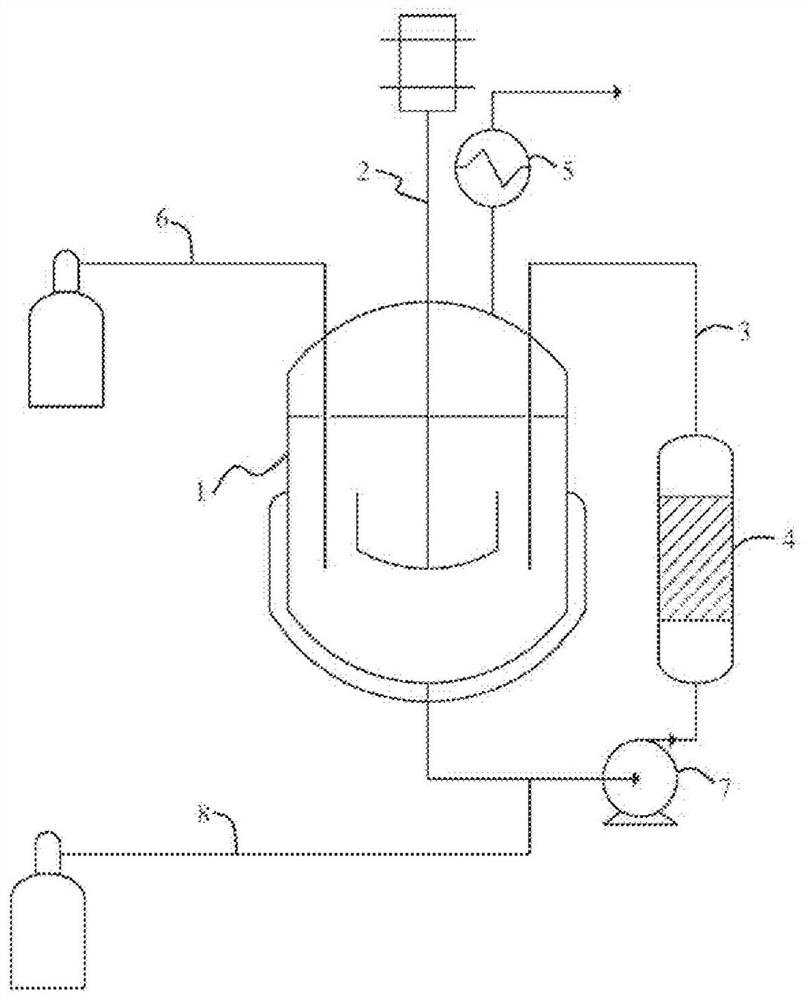
[0086] use figure 1 device indicated.
[0087] 300 g of DMF and 33.6 g (0.6 mol) of potassium hydroxide beads were introduced into a circulation loop (3) equipped with a stirrer (2) and a static mixer (4) and equipped with a reflux condenser (5) at the top ) in the 500ml reactor (1). Heating was carried out to 70° C. and sulfur dioxide gas was introduced through the dip tube ( 6 ) to obtain a concentration of 1% by weight in DMF. This concentration level was confirmed by in situ Raman analysis. When the concentration stabilized, gaseous CF was introduced under the pump suction of the circulation loop (7) via route (8) at a flow rate of 10 g / h 3 Br. Introducing CF 3 During this phase of Br, SO in the reactor 2 The concentration was kept at 1% by ...
example 2
[0089] Example 2 (comparison):
[0090] Repeat what is described in Example 1 for the preparation of CF 3 Br method, but with SO in DMF at 5 bar 2 The concentration was adjusted to 10% by weight to carry out the reaction. After analysis of the final crude reaction product, relative to the introduced CF 3 Br, CF 3 SO 2 The yield of K was 33%.
example 3
[0091] Example 3: According to the continuous implementation in SO 2 Preparation of trifluoromethane by sulfinating bromotrifluoromethane in the presence of sodium formate Sodium sulfinate CF 3 SO 2 Na
[0092] use figure 1 device indicated.
[0093] 300 g of DMF and 15 g (0.22 mol) of sodium formate powder were introduced into a 500 ml reaction equipped with a stirrer (2) and a circulation loop (3) equipped with a static mixer (4) and equipped with a reflux condenser (5) at the top device (1). Heating was carried out to 70° C. and sulfur dioxide gas was introduced through the dip tube ( 6 ) to obtain a concentration of 1% by weight in DMF. This concentration level was confirmed by in situ Raman analysis. When the concentration stabilized, gaseous CF was introduced under the pump suction of the circulation loop (7) via route (8) at a flow rate of 10 g / h 3 Br. Introducing CF 3 During this phase of Br, SO in the reactor 2 The concentration was kept at 1% by contin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com