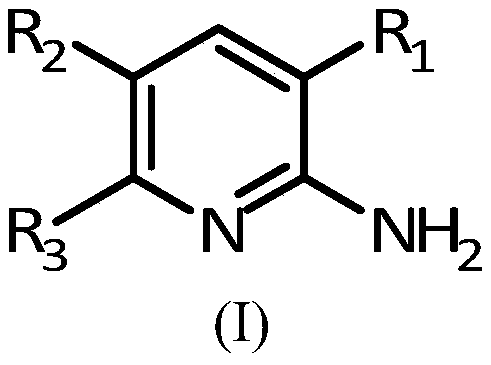
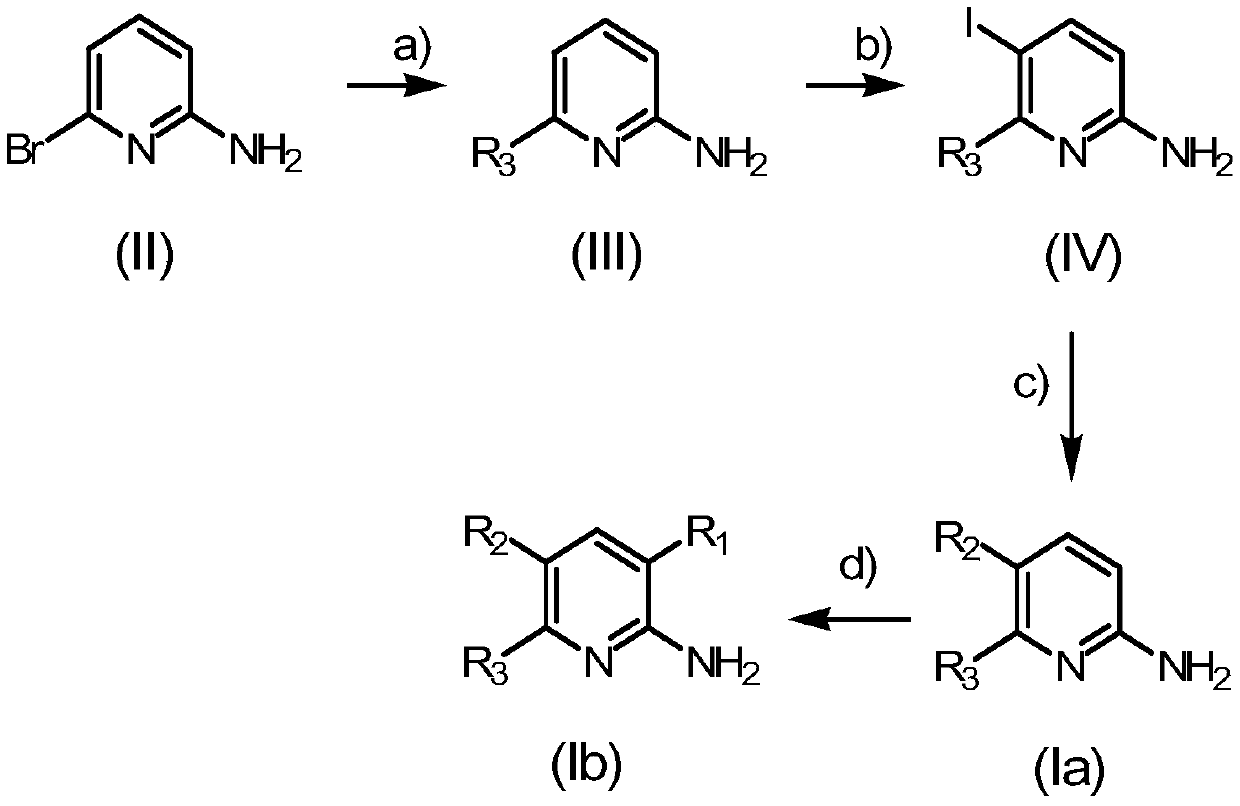
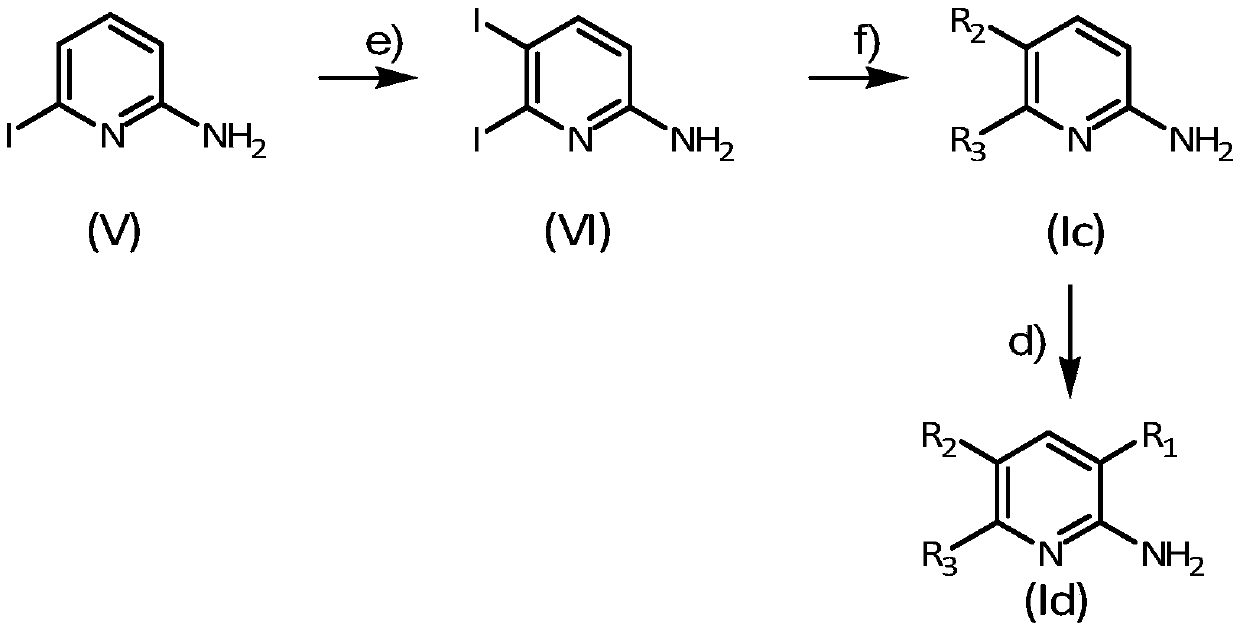
as adenosine a 2b Receptor antagonists and melatonin mt 3 2-aminopyridine derivatives of receptor ligands
A technology of pyridine and fluoropyridine, applied in the field of pyridine derivatives, can solve the problems of limiting the pro-inflammatory effect of adenosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Example 1 : 5-(pyridin-4-yl)-6-(pyrimidin-5-yl)pyridin-2-amine
[0263]
[0264] 5-Iodo-6-(pyrimidin-5-yl)pyridin-2-amine (0.53 g, 1.77 mmol) and (pyridin-4-yl)boronic acid pinacol ester (0.91 g, 4.42 mmoles), [1, 1'bis(diphenylphosphino)ferrocene]dichloropalladium(II) - complexed with dichloromethane (0.087g, 0.107mmoles), and 1,4-bis A mixture of 2M aqueous cesium carbonate solution (3.6 mL) in alkanes (14.3 mL) was heated to 110 °C and left to stir for 20 hours. The mixture was cooled and then partitioned between ethyl acetate and 1M aqueous sodium hydroxide solution. Partition the organic phase with sodium bicarbonate and brine. dry (MgSO 4) and evaporate the final organic layer. The residue precipitated as a pale pink fine solid which was rinsed with cold diethyl ether and dried (0.286 g, 54.8%).
[0265] 1 H-NMR (400MHz, DMSO-d 6 ): δ=6.52(s, 2H), 6.64(d, 1H), 7.13(d, 2H), 7.58(d, 1H), 8.44(d, 2H), 8.61(s, 2H), 9.09(s, 1H).
[0266] HPLC-MS: Rt 1.88...
Embodiment 2
[0267] Example 2 : 5-(3-fluoropyridin-4-yl)-6-(pyrimidin-5-yl)pyridin-2-amine
[0268] Following the procedure described in Example 1, this product was synthesized from 6-(pyrimidin-5-yl)pyridin-2-amine and 3-fluoropyridine-4-boronic acid.
[0269] 1 H-NMR (400MHz, DMSO-d 6 ): δ=6.61(s, 2H), 6.65(d, 1H), 7.38(dd, 1H), 7.57(d, 1H), 8.39(d, 1H), 8.43(d, 1H), 8.62(s, 2H), 9.10(s, 1H).
[0270] HPLC-MS: Rt 2.082m / z 268.0 (MH + ).
Embodiment 3
[0271] Example 3 : 6-(pyridin-3-yl)-5-(pyridin-4-yl)pyridin-2-amine
[0272] Following the procedure described in Example 1, this product was synthesized from 6-(pyridin-3-yl)pyridin-2-amine and pyridine-4-boronic acid.
[0273] 1 H-NMR (400MHz, DMSO-d 6 ): δ=6.42(s, 2H), 6.59(d, 1H), 7.06(d, 2H), 7.30(dd, 1H), 7.55(d, 1H), 7.63(d, 1H), 8.37(d, 1H), 8.39(d, 2H), 8.46(d, 1H).
[0274] HPLC-MS: Rt 2.804m / z 249.1 (MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com