Cisplatin-flurbiprofen prodrug, as well as preparation method and application thereof
A flurbiprofen and cisplatin technology, applied in the field of chemical prodrugs and their preparation, can solve the problems of not reversing drug resistance, unable to more effectively improve drug treatment effect, etc., and achieve the effect of enhancing anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
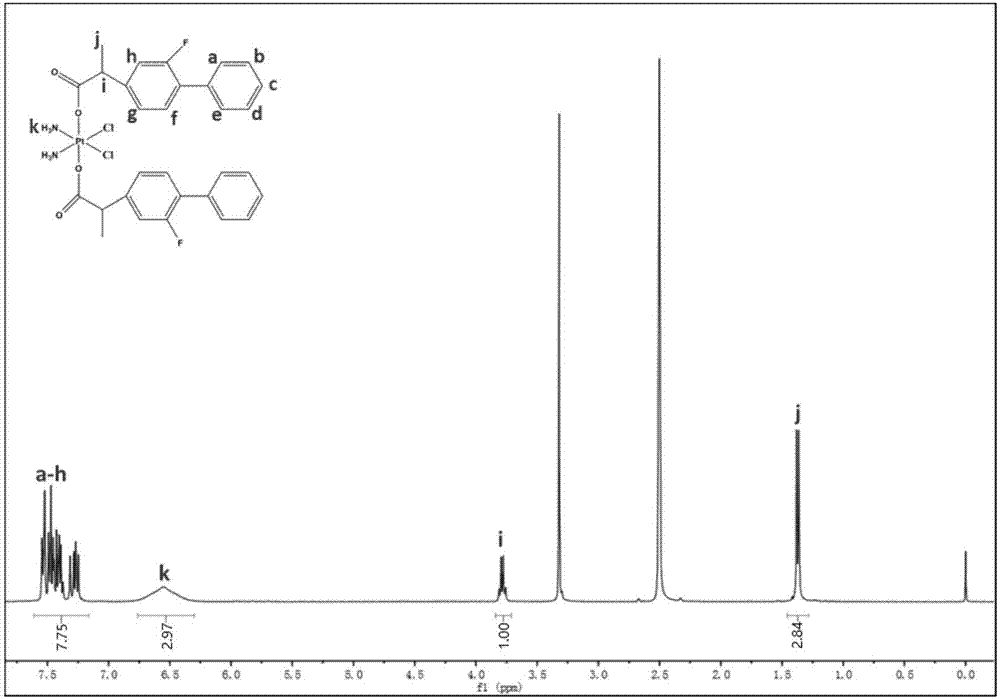
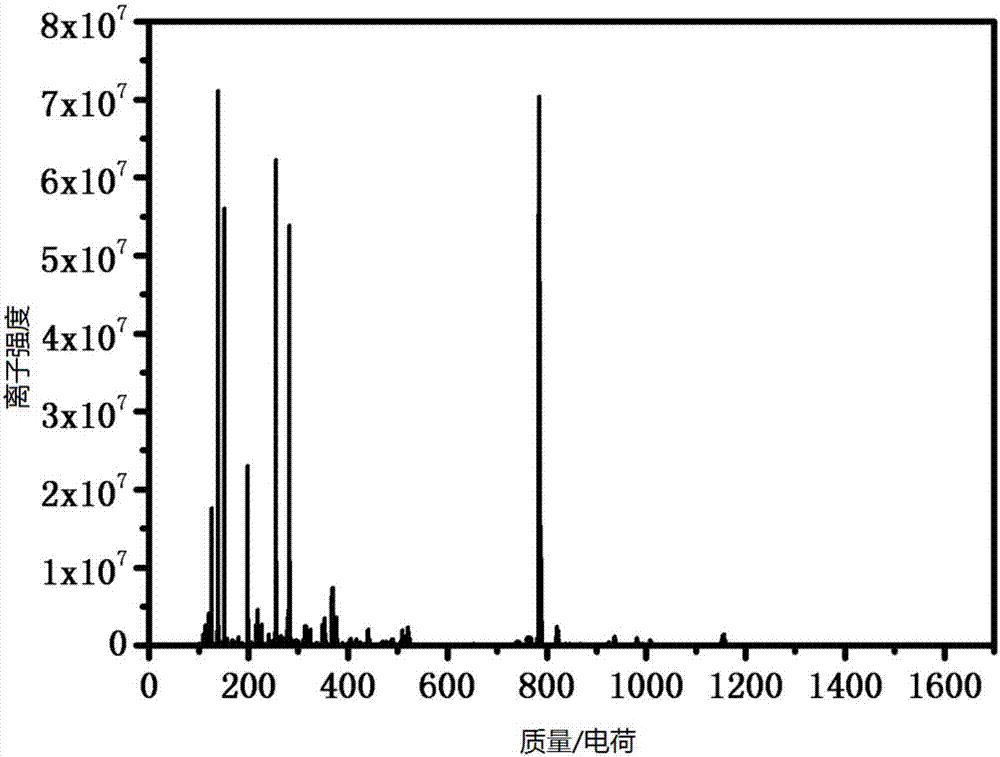
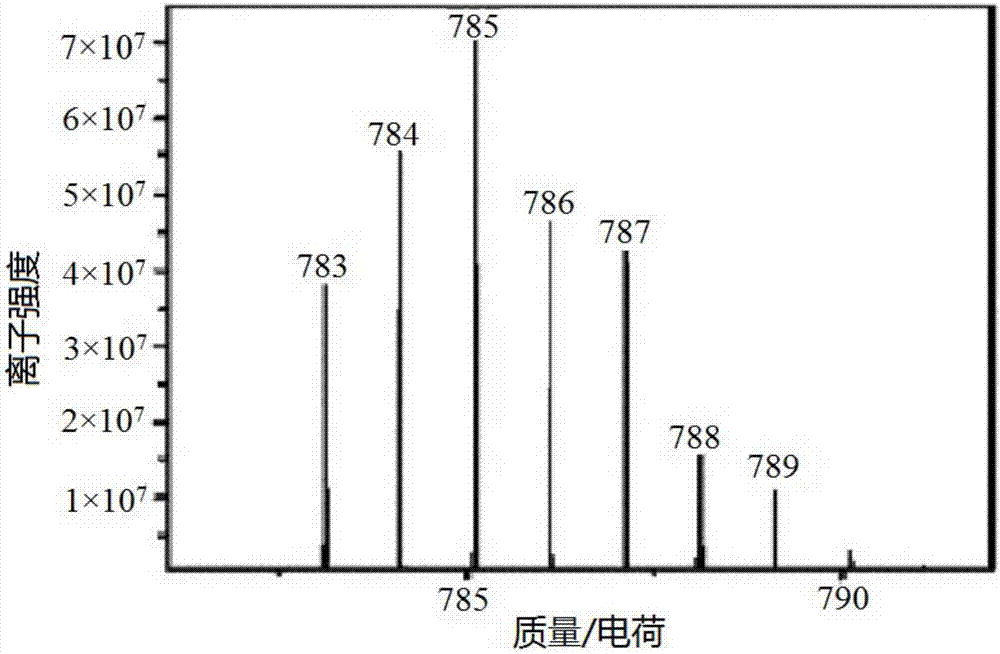
[0035] This example prepares cisplatin-flurbiprofen prodrug by the method comprising following steps.
[0036] (1) Add 1005.94mg (4.12mmol) flurbiprofen in the three-necked flask, pass N 2 half an hour.
[0037] (2) Add oxalyl chloride into the three-necked flask (so that the molar ratio of flurbiprofen to oxalyl chloride is 1:10), heat up to 70°C, heat, stir, and reflux for 1 hour, and cool to room temperature to obtain a yellow transparent liquid.
[0038] (3) Transfer the yellow transparent liquid obtained in step (2) to an eggplant-shaped bottle, and remove the oxalyl chloride by rotary evaporation to obtain a yellow oily liquid; add 6 mL of anhydrous tetrahydrofuran (THF), and remove it by rotary evaporation; repeat this step twice .
[0039] (4) Add 69.3mg (0.207mmol) dichlorodihydroxydiammine platinum (Pt(NH 3 ) 2 (OH) 2 Cl 2), 6mL tetrahydrofuran (THF), under the condition of avoiding light, heat up to 70°C, heat, stir, and reflux for 2h, a light yellow precipita...
Embodiment 2
[0047] This example prepares cisplatin-flurbiprofen prodrug by the method comprising following steps.
[0048] (1) Add 1005.94mg (4.12mmol) flurbiprofen in the three-necked flask, pass N 2 half an hour.
[0049] (2) Add oxalyl chloride into the three-necked flask (so that the molar ratio of flurbiprofen to oxalyl chloride is 1:20), heat up to 70°C, heat, stir, and reflux for 2 hours, and cool to room temperature to obtain a yellow transparent liquid.
[0050] (3) Transfer the yellow transparent liquid obtained in step (2) to an eggplant-shaped bottle, and remove the oxalyl chloride by rotary evaporation to obtain a yellow oily liquid; add 5 mL of tetrahydrofuran (THF), and remove it by rotary evaporation; repeat this step twice.
[0051] (4) Add 46.0mg (0.137mmol) dichlorodihydroxydiammine platinum (Pt(NH 3 ) 2 (OH) 2 Cl 2 ), 6mL tetrahydrofuran (THF), under the condition of avoiding light, the temperature was raised to 70°C, heated, stirred, and refluxed for 3h, a light ...
Embodiment 3
[0057] This example prepares cisplatin-flurbiprofen prodrug by the method comprising following steps.
[0058] (1) Add 1005.94mg (4.12mmol) flurbiprofen in the three-necked flask, pass N 2 half an hour.
[0059] (2) Add oxalyl chloride into the three-necked flask (so that the molar ratio of flurbiprofen to oxalyl chloride is 1:4), heat up to 50°C, heat, stir, and reflux for 0.5h, and cool to room temperature to obtain a yellow transparent liquid.
[0060] (3) Transfer the yellow transparent liquid obtained in step (2) to an eggplant-shaped bottle, and remove the oxalyl chloride by rotary evaporation to obtain a yellow oily liquid; add 6 mL of tetrahydrofuran (THF), and remove by rotary evaporation; repeat this step twice.
[0061] (4) Add 138.6mg (0.414mmol) dichlorodihydroxydiammine platinum (Pt(NH 3 ) 2 (OH) 2 Cl 2 ), 6mL tetrahydrofuran (THF), under the condition of avoiding light, heat up to 50°C, heat and stir for 1h, a light yellow precipitate is obtained in the bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com