A kind of furatriptan succinate sustained-release microspheres and preparation method thereof
A technology of succinic acid and furotril, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of high recurrence rate, short half-life, inconvenient to carry and use, etc. , to achieve good treatment effect, improve patient compliance, and enhance the effect of curative effect
Active Publication Date: 2019-10-01
CP PHARMA QINGDAO CO LTD
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
As a second-generation triptan drug, it overcomes the disadvantages of low oral bioavailability, short half-life and high relapse rate of the first-generation 5-HT1B / 1D receptor agonists
At present, there are mainly furovatriptan succinate tablets and injections on the market in our country. Although conventional tablets are convenient to take, they are affected by factors such as disintegration and drug release, and the absorption effect is not ideal. multiple doses
Although the curative effect of the injection is fast, it is inconvenient to carry and use.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Login to View More
Abstract
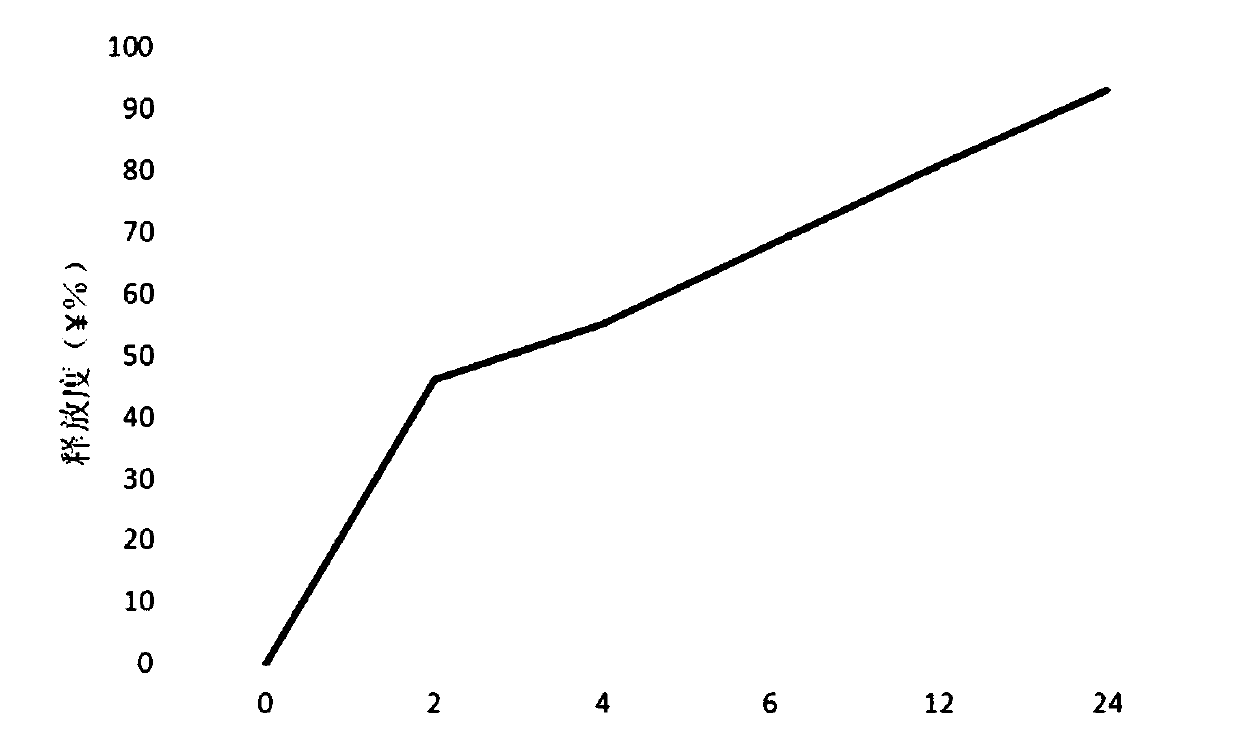
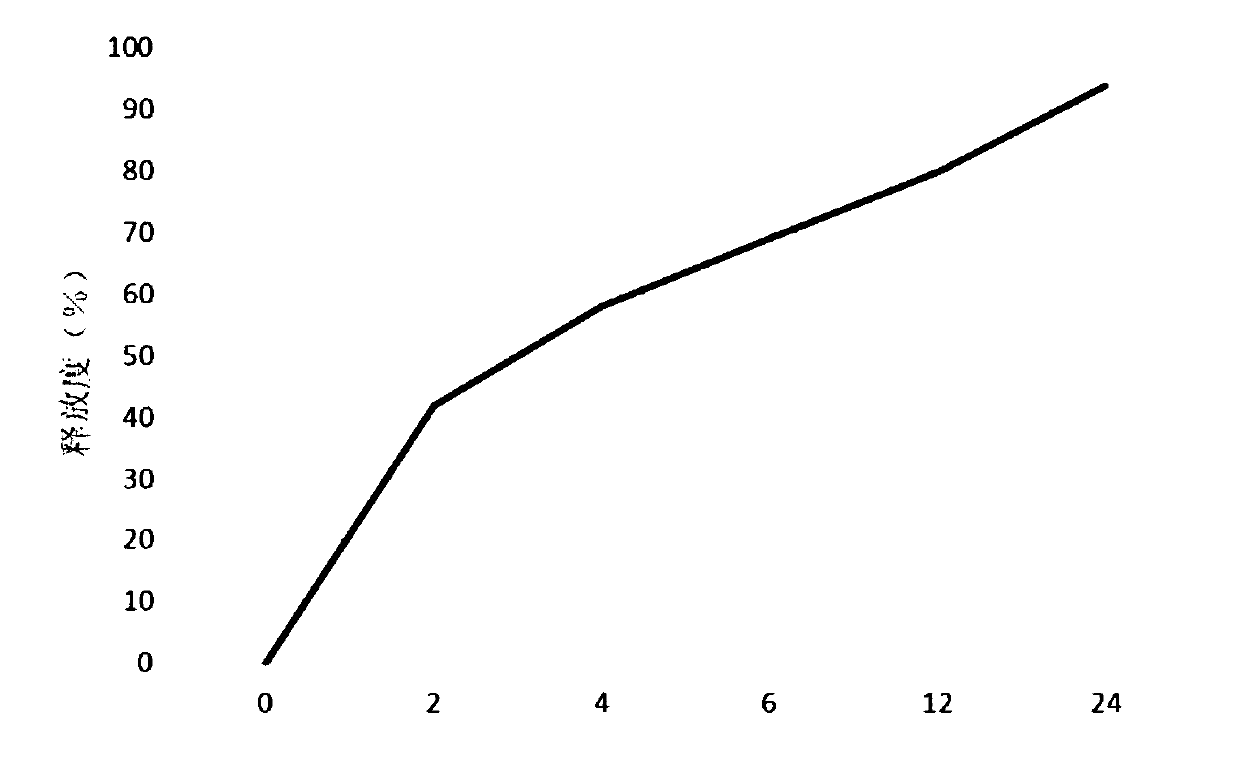
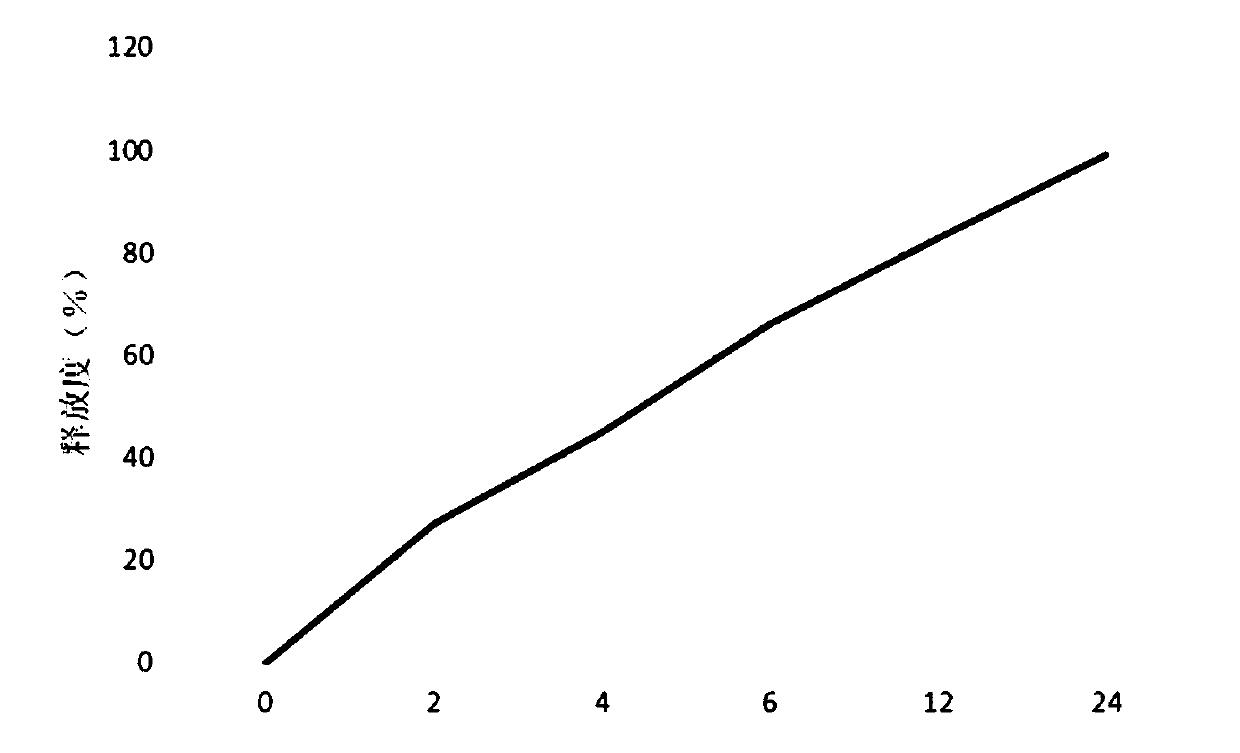
The invention provides a frovatriptan succinate sustained-release microsphere, which consists of frovatriptan succinate and PLGA, wherein the weight ratio of the frovatriptan succinate to the PLGA is at 1: (1-15); and the frovatriptan succinate accounts for 15.9-23.7% in terms of the total weight of the microsphere, wherein in the PLGA, the molar ratio of lactic acid to glycolic acid is at (75-50): (25-50). With the application of the microsphere, an effective blood concentration can be kept for a relatively long time, the compliance of patients can be improved and a curative effect can be enhanced; and importantly, the microsphere is safe and controllable.
Description
Technical field The invention relates to a sustained-release furotriptan succinate preparation and a preparation method thereof. Background technique Furotriptan succinate, also called frovatriptan succinate, Chinese chemical name (R)-3-(methylamino)-2,3,4,9-tetrahydro-1H-carbazole-6- Formamide succinic acid. Furotriptan succinate is a new anti-migraine drug. This product mainly acts on the extracerebral arteries and intracranial arteries, and inhibits the excessive expansion of these blood vessels. As a second-generation triptan drug, it overcomes the shortcomings of the first-generation 5-HT1B / 1D receptor agonist of low oral bioavailability, short half-life and high recurrence rate. At present, there are mainly furotriptan succinate tablets and injections on the market in my country. Although conventional tablets are convenient to take, they are affected by factors such as disintegration and drug release. The absorption effect is not ideal, and they are often needed in the ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/52A61K31/403A61K47/34A61K47/32A61K47/04A61P25/06
Inventor 陈阳生王明刚刘晓霞孙桂玉臧云龙
Owner CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com