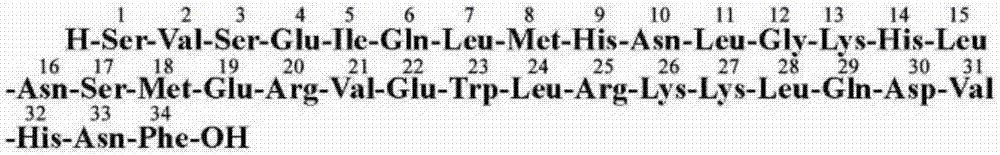Method for preparing teriparatide
A technology of teriparatide and synthesis method, which is applied in the field of preparation of teriparatide, can solve the problems of only 32.2% total yield, large content of teriparatide, complicated work and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
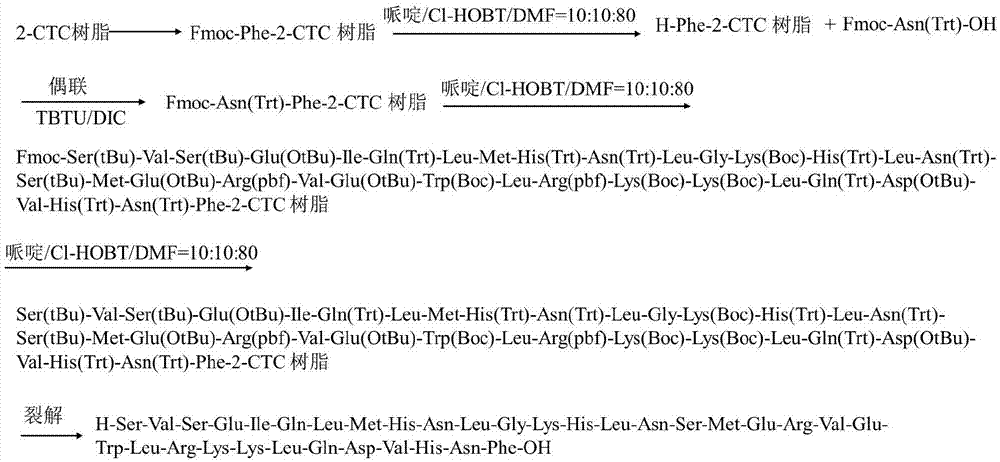
[0075] (1) Preparation of Fmoc-Phe-CTC Resin resin
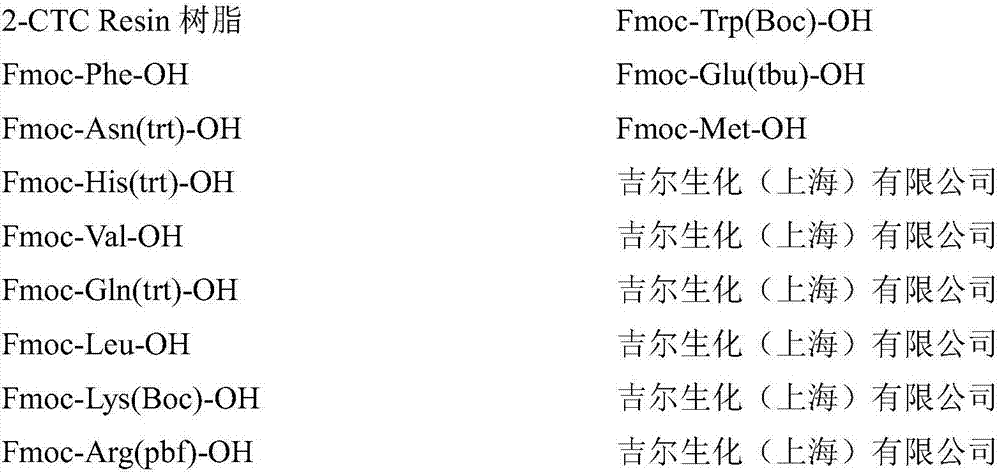
[0076] Add 20g (28 mmol) of CTC Resin resin with a substitution degree of 1.4mmol / g into the glass reaction column, add 200mL of DCM to dissolve it for 20min, drain it, and repeat 3 times; at the same time, weigh 10mmol of Fmoc-Phe-OH and 60 mmol of DIEA, these two substances were dissolved in 200 mL of DCM, stirred evenly, poured into a glass reaction column, and stirred for 1 hour. Extract liquid thereafter, wash with DCM, wash 6 times, each consumption 200mL, then wash with DMF, wash 6 times, each consumption 200mL; promptly obtain Fmoc-Phe-CTC Resin resin 45.1g, substitution degree is 0.4mmol / g.
[0077] (2) Synthesis of full peptide resin
[0078] To the Fmoc-Phe-CTC Resin resin that step 1) generates, add the DMF solution of 20% piperidine that the volume concentration that 400mL configures is good, stir and make it react for 30min, extract liquid afterwards, wash product 6 times with DMF, every Use 200mL each time,...
Embodiment 2
[0099] 1) Take 3.3g of Fmoc-Phe-wang-Resin resin with a degree of substitution of 0.3mmol / g and add 20mL of DMF solution of piperidine with a volume concentration of 20% and stir it to react for 30min, then remove the liquid and use DMF Wash the product 6 times, each time 20mL, drain the washing solution DMF, then weigh 1mmol of Fmoc-Asn(trt)-OH, 1mmol of TBTU and 1mmol of HOBT, and use 20mL of DMF for these three substances: DCM = 1:1 mixed solvent dissolved, after the dissolution is complete, add 10mmol of DIEA to form a mixed solution, stir evenly again, add the mixed solution into the glass reaction column, stir for 2 hours, then remove the liquid, and use DMF to prepare the product Wash 6 times, each time with 20 mL, and drain to obtain 5.1 g of Fmoc-Asn(trt)-Phe-wang Resin resin.
[0100] 2) Take half of the above resin, add piperidine / DMF solution with a volume ratio of 1:4 to remove the Fmoc protecting group on the resin, react for 30min, add 1mmol of Fmoc-His(trt)-OH,...
Embodiment 3
[0104] 1) Take 3.3g of Fmoc-Phe-wang-Resin resin with a degree of substitution of 0.3mmol / g and add 20mL of DMF solution of piperidine with a volume concentration of 20% and stir it to react for 30min, then remove the liquid and use DMF Wash the product 6 times, each time 20mL, drain the washing solution DMF, then weigh 1mmol of Fmoc-Asn(trt)-OH, 1mmol of TBTU and 1mmol of HOBT, and use 20mL of DMF for these three substances: DCM = 1:1 mixed solvent dissolved, after the dissolution is complete, add 10mmol of DIEA to form a mixed solution, stir evenly again, add the mixed solution into the glass reaction column, stir for 2 hours, then remove the liquid, and use DMF to prepare the product After washing 6 times, the dosage of each time was 20 mL, and then drained to obtain 5.1 g of Fmoc-Asn(trt)-Phe-wang-Resin resin.
[0105] 2) Take half of the above resin, remove the Fmoc protecting group on the resin with a deprotecting agent with a volume percentage concentration of 1:1:8 pip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com