Tissue-derived exosome extraction method and application thereof
An extraction method and exosome technology, which are applied in the field of tissue-derived exosome extraction to achieve the effect of convenient operation and field expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
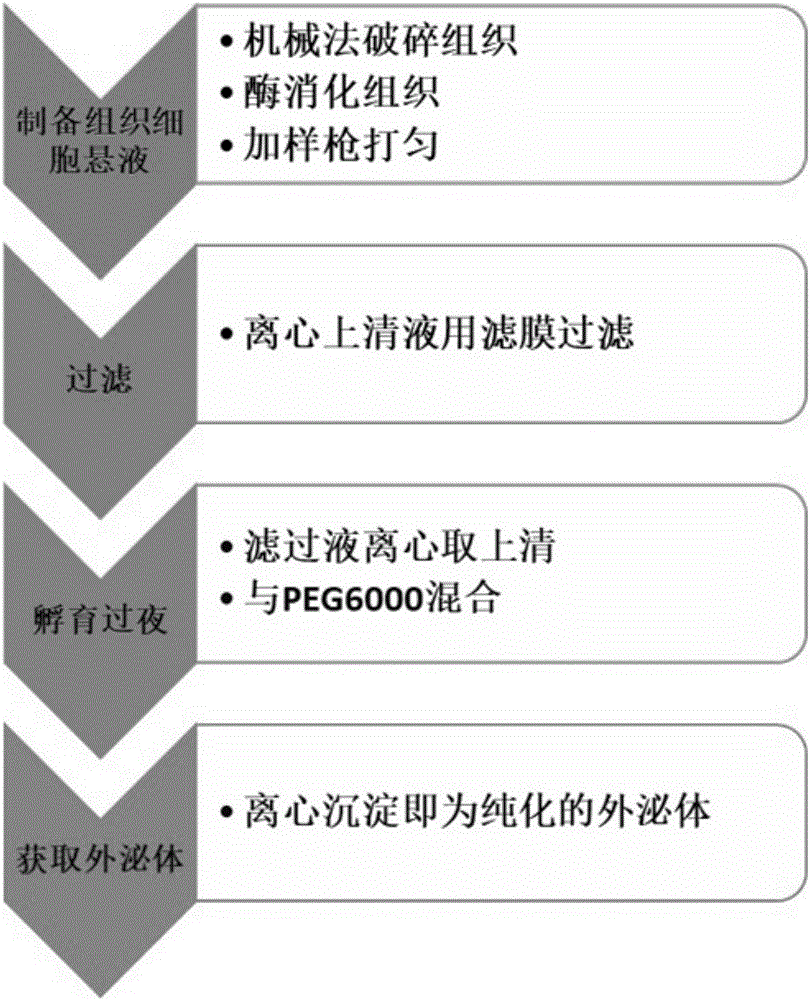
[0035] 1. Place the fresh tissue block in 350μl 1640 medium pre-cooled;
[0036] 2. Mince the tissue block into 1mm pieces with a sharp blade 3 size, operated on ice;
[0037] 3. Add 350 μl of 1 mg / ml collagenase IV and 2 μl of 0.2% (w / v) Dnase I, cut off the sharp part of the pipette tip, and gently pipette the mixture;
[0038] 4. Digest the above mixture on a constant temperature shaker at 37°C and 100 rpm for 90 minutes until the tissue pieces are invisible to the naked eye, then stop the digestion, and collect the samples at 4°C to delay their digestion;
[0039] 5. After all the tissue pieces are completely digested, transfer to a test tube, centrifuge at 3000g 4°C for 30min, absorb the supernatant, transfer to a new centrifuge tube, centrifuge at 13000g 4°C for 10min, and harvest the supernatant;
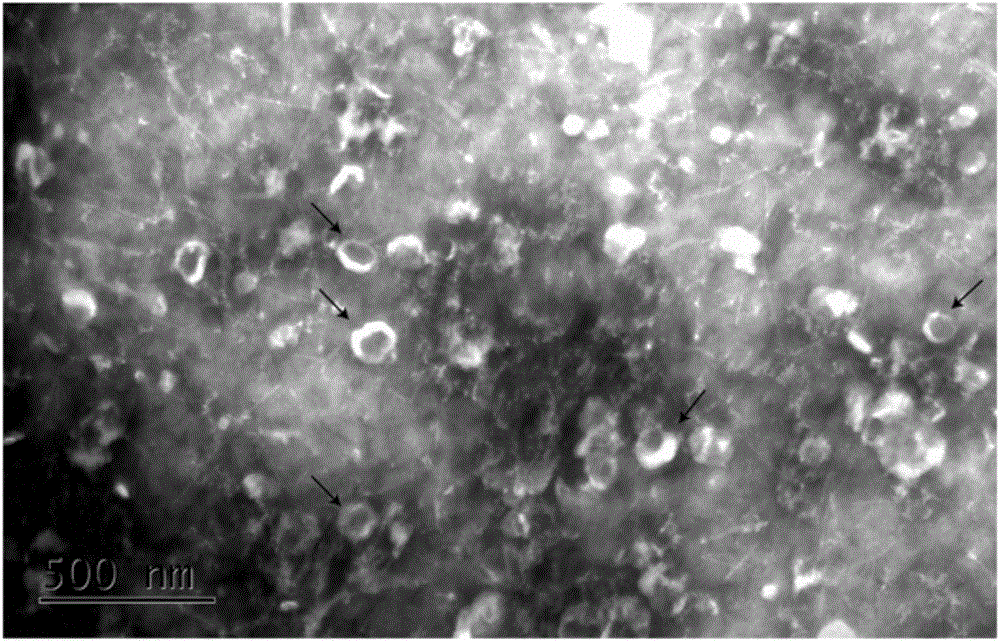
[0040] 6. Pass the supernatant through a 0.22μm filter, transfer the filtrate to a new centrifuge tube, centrifuge at 13,000g at 4°C for 10min, and collect the supernatant;...
Embodiment 2
[0049] 1. Place the cryogenically frozen (-80°C) tissue block in 350 μl 1640 medium pre-cooled (frozen tissue needs to be quickly placed in a 37°C water bath for 5 minutes);
[0050] 2. Mince the tissue block into 1mm pieces with a sharp blade 3 size, operated on ice;
[0051] 3. Add 350 μl of 1 mg / ml collagenase IV and 2 μl of 0.2% (w / v) Dnase I, cut off the sharp part of the pipette tip, and gently pipette the mixture;
[0052] 4. Digest the above mixture on a constant temperature shaker at 37°C and 100 rpm for 80 minutes until the tissue pieces are invisible to the naked eye, then stop the digestion, and collect the samples at 4°C to delay their digestion;
[0053] 5. After all the tissue pieces are completely digested, transfer to a test tube, centrifuge at 3000g 4°C for 30min, absorb the supernatant, transfer to a new centrifuge tube, centrifuge at 13000g 4°C for 10min, and harvest the supernatant;
[0054] 6. Pass the supernatant through a 0.22μm filter, transfer the fil...
Embodiment 3
[0058] 1. Place cryogenically frozen (liquid nitrogen) tissue pieces in pre-cooled 350 μl 1640 medium (frozen tissues need to be quickly placed in a 37°C water bath for 5 minutes);
[0059] 2. Mince the tissue block into 1mm pieces with a sharp blade 3 size, operated on ice;
[0060] 3. Add 350 μl of 1 mg / ml collagenase IV and 2 μl of 0.2% (w / v) Dnase I, cut off the sharp part of the pipette tip, and gently pipette the mixture;
[0061] 4. Digest the above mixture on a constant temperature shaker at 37°C and 100rpm for 100 minutes until the tissue pieces are invisible to the naked eye, then stop the digestion, and collect the samples at 4°C to delay their digestion;
[0062] 5. After all the tissue pieces are completely digested, transfer to a test tube, centrifuge at 3000g 4°C for 30min, absorb the supernatant, transfer to a new centrifuge tube, centrifuge at 13000g 4°C for 10min, and harvest the supernatant;
[0063] 6. Pass the supernatant through a 0.22μm filter, transfe...
PUM
| Property | Measurement | Unit |
|---|---|---|
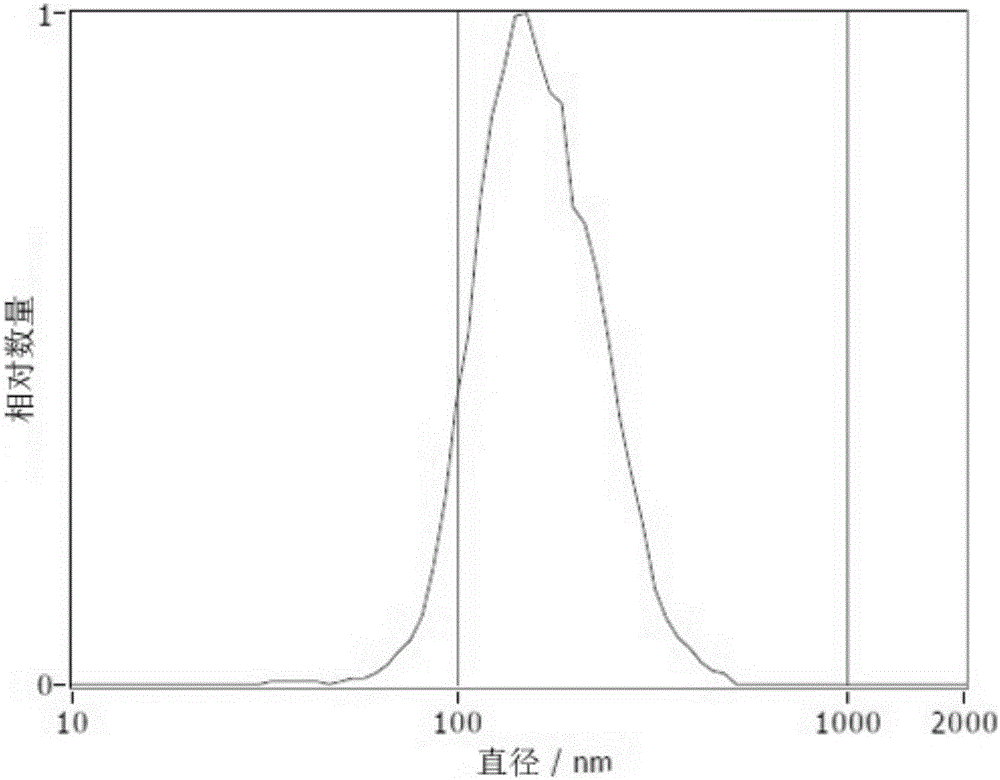
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com