Pefloxacin mesylate injection
A technology of pefloxacin mesylate and injections, which is applied in drug delivery, inorganic non-active ingredients, antibacterial drugs, etc., and can solve problems such as increased allergies, darker solution color, and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0051] Pefloxacin sulfonate 400g (calculated as pefloxacin)
[0052] Sodium thiosulfate 2.5g
[0053] Activated carbon 7.5g
[0054] Add water for injection to 5000ml
[0055] Made 1000 pieces
[0056] making process:
[0057] 1. Take the prescribed amount of sodium thiosulfate and place it in a concentrated tank, add 75% water for injection to dissolve. Self-made 0.5mol / L NaOH solution for subsequent use;
[0058] 2. Take the prescribed amount of pefloxacin mesylate and add it to the solution to dissolve it;
[0059] 3. Use 0.5mol / L NaOH solution to adjust the pH between 4.5 and 5.0;
[0060] 4. Add water for injection and make the volume to 5000ml;
[0061] 5. When the temperature drops to 50°C, add activated carbon and stir for 30 minutes;
[0062] 6. Filter with a 0.45um filter membrane first, and then filter with a 0.22um filter membrane;
[0063] 7. Potting and sealing, the finished product is sterilized at 115°C for 30 minutes.
[0064] 8. In the above process...
experiment example
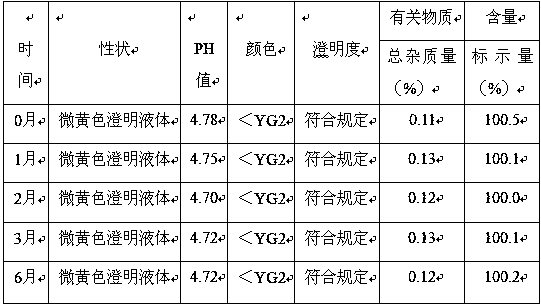
[0079]Experimental example: Take the above sample and store it under the condition of 40℃±2℃ / RH75±5% for six months, and take a sample at the end of the first, second, third and sixth months respectively, according to the second part of "Chinese Pharmacopoeia" The pefloxacin mesylate injection standard was used as the basis for investigation, and the properties, acidity, color, clarity, related substances (such as unknown impurities), content, etc. were tested, and the results were compared in October. See Table 1 for the results
[0080] Table 1
[0081]
[0082] The result shows: the color of the sample of the present invention is slightly yellow when the accelerated test is carried out for 6 months, and meets the standard of the Pharmacopoeia. At the same time, the change of its pH value is not large, within the fluctuation range. The total impurity <0.5%, and the content does not change much. Therefore, the samples produced under this prescription process have good s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com